This is the latest version of this chapter. The previous version can be found here.
Stem cells have added a new thrust to tissue engineering. Their distinctive self-renewal and plasticity have not only optimized many tissue engineering developments, but also rendered feasible some applications which would otherwise be unattainable with somatic cells. This review focuses on general aspects of autologous tissue engineering based on so-called adult stem cells, which not only allow for a number of the therapeutic strategies envisioned for reprogrammed and embryonic stem cells, but also tend to be genetically more stable than the latter, not to mention less restrained by regulatory hurdles and ethical controversy. While the breadth of stem cell-based tissue engineering has yet to be defined in various large scale clinical applications, numerous experimental reports and the early human experience underwrite its potential. The field continues to advance robustly in an ever expanding number of laboratories around the world, yet much remains to be learned and developed, not only by scientists and clinicians, but also entrepreneurs and regulatory agencies. Nevertheless, given its scientific premises, the potential magnitude of its impact to society, and what has been achieved thus far, the perspective of stem cell-based tissue engineering reaching the mainstream of clinical practice has become a justifiable realistic expectation.
1. Introduction
The promise of tissue engineering has predictably gained renewed impetus with the addition of stem cells to its armamentarium. While the broader definition of tissue engineering includes a few non cell-based methods, the vast majority of its processes comprise different forms of cell therapies, for which stem cells, with their distinctive self-renewal and plasticity, are particularly suitable. In addition, stem cells allow for certain engineering strategies that would be impossible with other cell types. For example, the fact that it has been difficult to expand a sizeable proportion of somatic cells in sufficiently large numbers ex vivo for the engineering of many tissues or organ substitutes can, often times, be overcome by the use of stem cells. Indeed, the virtually limitless conceptual viability of autologous approaches to tissue engineering is one of the most direct consequences of this very principle.
While the breadth of stem cell-based tissue engineering has yet to be defined in different large scale clinical applications, numerous experimental reports and the initial clinical experience underwrite its potential. Still, perhaps the most compelling substantiation of this principle is nature's numerous precedents of tissue regeneration stemming from naturally-occurring (tissue-specific or not) stem cell activity. Indeed, the biomimetic stimulation of native stem cells has been an actively pursued strategy in regenerative medicine for some time now. This concept, however, is beyond the scope of this chapter. Other alternative approaches equally beyond this review include stem cell-mediated gene therapy; tolerance induction; embryonic stem cells; somatic cell nuclear transfer; and induced pluripotent stem cell (iPS) reprogramming as autologous approaches to stem cell-based tissue engineering – all of which are covered in other chapters. This review focuses on general aspects of tissue engineering based on so-called adult stem cells, which not only allow for a number of the therapeutic strategies envisioned for reprogrammed and embryonic stem cells, but also tend to be genetically more stable than the latter, as well as disconnected from their specific regulatory hurdles and eventual ethical controversy.
2. Cell sources
Different putative sources of adult stem cells have been identified or suggested almost regularly over the last several years. The search for alternative sites from which to isolate these cells and/or for new means to obtain them has been one of the most prolific aspects of the whole field, with an abundance of reports to date. Adult progenitor cells have been isolated from bone marrow (Pittenger, Mackay et al. 1999; Jiang, Jahagirdar et al. 2002), peripheral blood (Amos and Gordon 1995; Smith and Storms 2000), umbilical cord blood (Amos and Gordon 1995; Romanov, Svintsitskaya et al. 2003), placenta (Rubinstein, Carrier et al. 1998; Fauza 2004), amniotic fluid (Torricelli, Brizzi et al. 1993; Kaviani, Perry et al. 2001; Prusa and Hengstschlager 2002; Fauza 2004; Tsai, Hwang et al. 2006), amniotic membrane (Sakuragawa, Enosawa et al. 2000; Takahashi, Enosawa et al. 2002), Wharton jelly (Wang, Hung et al. 2004), adipose tissue (Zuk, Zhu et al. 2001; Wagner, Wein et al. 2005; Kern, Eichler et al. 2006), dermis (Toma, Akhavan et al. 2001; Young, Steele et al. 2001), hair follicle (Amoh, Li et al. 2005), synovial membrane (De Bari, Dell’Accio et al. 2001), skeletal muscle (Lee, Qu-Petersen et al. 2000; Young, Steele et al. 2001), central nervous system (Snyder, Deitcher et al. 1992), olfactory bulb (Liu and Martin 2004), retina (Coles, Angenieux et al. 2004), inner ear (Li, Liu et al. 2003), gastrointestinal epithelium (Bjerknes and Cheng 2002), fetal liver (Amos and Gordon 1995; Krupnick, Balsara et al. 2004), and likely others by the time this goes to print. Different progenitor cell types, including mesenchymal, epithelial, hematopoietic, neural, endothelial, and trophoblastic have been identified from all these sources, some of which harbor more than one type of progenitor cell. More recently, it has been shown that certain specialized stem cells can be isolated from certain sources in the presence of disease, such as for example the isolation of neural stem cells from amniotic fluid in the setting of neural tube defects (Turner, Klein et al. 2012). At the same time, however, despite the apparent surplus of reports on stem cell sources, many suffer from incomplete analyses and still require further in depth validation. Most of the more widely used cell isolation methods rely on different combinations of natural selection by the culture media with some form of direct cell isolation, typically mechanical, magnetic, or immune-based. In addition, oxygen tension manipultations, multi-stage cultures, and numerous alternative media formulations have also been explored, among many other variations of this principle.
The stem cell source can impact the algorithm for eventual clinical translation of a given tissue engineering strategy. Phenotypically comparable stem cells may behave quite differently, depending on the cell source considered (Wagner, Wein et al. 2005; Chang, Shih et al. 2006; Kern, Eichler et al. 2006; Kunisaki, Fuchs et al. 2007). Such differences can have a significant bearing not only on cell processing in vitro, but also on many relevant aspects of the engineered tissue made from comparable stem cells (Kunisaki, Fuchs et al. 2007). For example, mesenchymal stem cells (MSCs) procured from amniotic fluid proliferate significantly faster in vitro than immunophenotypically equivalent MSCs obtained from bone marrow or cord blood and lead to a very peculiar form of engineered cartilage, unusually rich in both glycosaminoglycans and α-elastin, when compared with constructs originated from these other MSCs, under equal bioreactor conditions (figure 1) (Kunisaki, Fuchs et al. 2007).
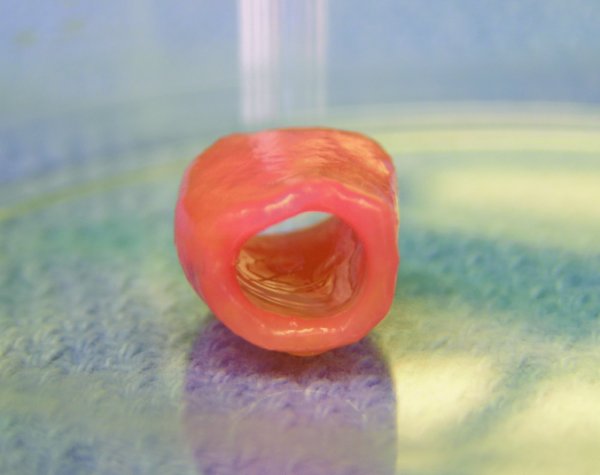
Typical gross appearance of a tubular cartilaginous construct engineered from amniotic mesenchymal stem cells.
The goal of generating clinically relevant engineered tissue places specific requirements on the cell type/source, including minimally invasive accessibility, the ability to produce an inordinately large quantity of cells in a relatively short period of time, hardiness to often prolonged in vitro processing, and reproducible differentiation pathways, among others. These parameters have favored certain stem cell types over others. Mesenchymal stem cells are among the most broadly utilized stem cells in tissue engineering due to their diverse sources, self-renewal pattern, and multilineage potential (Bianco and Robey 2001; Barrilleaux, Phinney et al. 2006). Indeed, most of the tissues used for structural surgical repair are mesenchymal in nature. Further, in addition to all mesenchymal lineages, certain MSCs such as those from umbilical cord blood and amniotic fluid can also differentiate into cells from different germ layers, sometimes all three of them. This broadens the appeal of these cells even further. While many sites have been shown to yield MSCs, the bone marrow remains the most studied and best characterized source, thus it is often the benchmark for comparisons involving MSCs. At the same time, nonetheless, MSC isolation and expansion from the bone marrow is more difficult than from other sources and can be significantly influenced by the donor's age (Vaananen 2005; Kunisaki, Fuchs et al. 2007). Indeed, “alternative” sources of MSCs, such as the amniotic fluid and adipose tissue, have recently received increasingly more attention for tissue engineering applications, due to their better translational appeal in many clinical scenarios, when compared with bone marrow and other sources (figure 2) (Kaviani, Perry et al. 2001; Kaviani, Guleserian et al. 2003; Awad, Wickham et al. 2004; Fuchs, Kaviani et al. 2004; Dragoo, Lieberman et al. 2005; Kunisaki, Freedman et al. 2006; Kunisaki, Fuchs et al. 2006; Kunisaki, Jennings et al. 2006; Kunisaki, Armant et al. 2007; Kunisaki, Fuchs et al. 2007; Stosich and Mao 2007; Steigman, Armant et al. 2008; Steigman, Ahmed et al. 2009; Klein, Turner et al. 2010; Turner, Klein et al. 2011; Gray, Turner et al. 2012; Turner, Klein et al. 2012).
3. Translational challenges
In order for novel therapeutic concepts and methodologies, such as those implicated in the development of autologous stem cell-based tissue engineering, to be brought to clinical fruition, many biological, technical, and regulatory hurdles must be overcome. While many of these hurdles are disease-specific, one can generalize certain aspects of the translational challenges involved.
3.1. Biological challenges
Timing is an intrinsic constraint in most tissue engineering concepts. Autologous construct-based approaches generally involve weeks, if not months, of processing ex vivo before final implantation. While the use of stem cells can often minimize this limitation, at least when compared with the use of most somatic cells, more often than not it remains a concern.
Even autologous cells may not be completely free of pathogens in culture, as the growth medium often requires xenogeneic products, such as fetal bovine serum. In addition, some cells can only propagate consistently on xenogeneic feeder layers, so that infectious risks cannot be completely eliminated.
The search for the ideal biocompatible scaffolding material(s) for a given clinical application is almost a never-ending pursuit. The possibility that newer components may prove better than what has been considered state of the art is an ever-present prospect. Many of the currently available synthetic scaffolds remain plagued by foreign body reaction in the setting of an immunocompetent environment, which in turn can lead to a reduction in the diffusion of nutrients and waste products, fibrosis, and other complications. Also, the cytotoxic effects of macrophage-generated nitric oxide can reach and destroy transplanted cells. Thus, it is not surprising that most of the scaffolds implanted in humans to date have been derived from natural sources. On the other hand, natural scaffolds are usually linked to unfavorable biomechanical properties and excessive heterogeneity. Also, chemicals used in decellularization processes often negatively affect different properties of the scaffold. Not surprisingly, the search for enhanced biocompatible synthetic biomaterials, such as elastomers, nanostructures, and others, remains a lively aspect of the development of tissue engineering, regardless of whether stem cell- or somatic cell-based. For example, scaffolds impregnated with select growth factors or specific peptide sequences may also allow for better control of the surrounding microenvironment. Undeniably, advances in synthetic materials will be instrumental in broadening the reach of tissue engineering both in the immediate and long term future.
3.1.1. Construct vascularization
The search for methods to establish and/or control the vascularization of engineered tissues or organs has been one of the major foci in the recent development of tissue engineering. Although the scaffold and three-dimensional milieu play a role, typically constructs greater than approximately 1cm in thickness cannot rely solely on vascular ingrowth from the host to remain viable in vivo (Davis, Schroeder et al. 2007). Thus, a major hurdle for clinical application of large engineered tissues and organs has been securing blood supply to the graft at the time of implantation. An appealing strategy among the many being currently explored to overcome this hindrance has been to build a microcirculation network within the engineered scaffold itself. One interesting example of such an approach has been the use of MicroElectroMechanical Systems (MEMS) (Fidkowski, Kaazempur-Mofrad et al. 2005). Advances in MEMS have enabled the development of a robust computational model of the vascular microcirculation, including variables such as fractal network topology, blood flow rheology, and the mass transfer of oxygen and nutrients across the vascular bed. This method has allowed the etching of vascular channels onto silicon wafers, which can then be transferred onto biodegradable polymer systems by stacking multiple monolayers of this architecture so as to form three-dimensional structures. Some of these strategies have already reached preclinical stage in large animal models (Hsu, Carraro et al. 2010). Another approach to organ revascularization has involved using the native vasculature of a decellularized organ for the seeding of donor endothelial cells in a bioreactor prior to anastomosis of the engineered graft, to date only attempted in rodent models (Ott, Clippinger et al. 2010). Various other approaches to establish or optimize construct vascularization, such as gene therapy, biomimetic microvascular guides and/or microfluidic networks, and scaffold-based delivery of angiogenic and/or growth factors, among others, have also been pursued (Wong Po Foo, Patwardhan et al. 2006; Brewster, Brey et al. 2007; Shen, Kastrup et al. 2008; Borenstein, Tupper et al. 2010; Loai, Yeger et al. 2010). Conceivably, many of the principles established by these developments might be also be eventually applied in facilitating the formation of other complex ancillary networks within engineered grafts, such as neural, lymphatic, and biliary systems, for example (Allmeling, Jokuszies et al. 2006).
4. Regulatory challenges
The Food and Drug Administration (FDA) has mandatory jurisdiction over cell-based therapies in The United States, including tissue engineering. An elaborate and costly infrastructure is necessary for the development and manufacture of engineered tissue amenable to FDA validation. Such regulatory clearance demands the use of so-called Good Manufacturing Practice (GMP) facilities, which not only must fulfill strict physical and operational requirements, but also be controlled by a critical mass of highly trained personnel. Another practical intricacy is the fact that certain tissues require preconditioning in complex bioreactors, which may not be readily compatible with large scaled manufacturing and shipping (Griffith and Naughton 2002). All of these underlying hurdles translate into chronic difficulties in establishing multicentric clinical trials, which are essential for the widespread application of technologies such as this.
Regulatory constraints have significantly hampered the clinical translation of many tissue engineering therapies (Lee, Arcidiacono et al. 2010). The FDA has often been criticized for slow approval processes, which include both justifiable and debatable requirements. This predicament has been attributed, at least to some extent, to the lack of clear, predictable regulatory frameworks and to uncertainties regarding the proper classification of different tissue engineered products. This is particularly evident when stem cells are to be used, in that, besides eventual ethical concerns, they typically trigger regulatory demands for unique safety data sets, more notably on genomic stability and tumorigenesis. Fortunately, it seems that the FDA is mindful of this scenario and is working to address these concerns. Meanwhile, however, many American biotechnology companies have engaged in collecting clinical data overseas, at lower costs, as most other countries have less stringent regulatory procedures (Atala, Bauer et al. 2006; McAllister, Maruszewski et al. 2009).
Regardless, these constraints, combined with strict reimbursement policies and poor business models, have typically rendered tissue engineering products commercially unsustainable in the long run (Fauza 2003). Indeed, a sizeable proportion of firms with considerable interest in tissue engineering have exited the market over the last 10 plus years. On the other hand, a number of companies still continue to invest in the development of new products (Lysaght and Reyes 2001; Vacanti 2008). It seems that a healthy partnership between academia and industry should be further explored as a means to expand tissue engineering into clinical reality on a more meaningful scale, a principle which has been proven viable in a number of circumstances (Fauza 2003; Vacanti 2008).
5. Current clinical applications
The infancy of the field, combined with the different challenges briefly discussed above, elucidate why few controlled prospective trials have validated clinical tissue engineering applications to date. Indeed, over the last decade a number of tissue-engineered products have either been abandoned following phase I/II clinical trials, or have failed in phase III clinical testing (Lysaght and Hazlehurst 2004). Very many challenges remain to be overcome by companies before “off-the-shelf” tissues can be offered commercially, including adequate sources of healthy expandable cells, the optimization of scaffolds, scaled-up bioreactors, the prevention of tissue rejection, and optimal product preservation ex vivo. Many of these challenges can be better tackled by the use of stem cells. Perhaps the best and most successful example is hematopoietic stem cell transplantation, which, first performed in humans during the 1950s, has long been firmly established clinically and will not be further explored here. Below is a brief yet illustrative sampling of some of the recent clinical experiences with tissue engineering. It is by no means purported to be a comprehensive, all-inclusive list. In that regard, we must remember that, although some still link the term “tissue engineering” solely to the paradigm of cell delivery within biocompatible scaffolds, the field is certainly much broader (Langer and Vacanti 1993; Vacanti and Langer 1999). In essence, tissue engineering technologies fall into one of four main strategies: delivery of isolated/expanded cells (simple cell transfers); tissue-inducing substances (not applicable to this review); extracorporeal and encapsulation techniques (closed systems); and cell transplantation within three-dimensional matrices (open systems).
5.1. Cardiovascular repair
End stage congestive heart failure and myocardial infarction remain the leading causes of death in the United States. The underlying process in these forms of cardiac disease seems related to boss a loss of functional cardiomyocites and to the inability of the myocardium to regenerate. The myocardium cannot repair itself at least in part because of a paucity of myocardial stem cells, not to mention the disease process and the consequential local hostile environment. The end result is fibrotic scarring, resulting in a decrease in the ventricular ejection fraction, diastolic dysfunction, and an overall decline in mechanical performance.
Over the last several years, the local delivery of autologous skeletal muscle satellite cells or bone marrow-derived stem cells, a procedure known as cellular cardiomyoplasty, has been evaluated as an experimental therapy in patients with ischemic myocardium. To date, hundreds of patients have received this therapy worldwide. This approach aims to minimize fibrotic remodeling of the injured myocardium by populating the damaged area with myogenic precursor cells. Support for this hypothesis has been based largely on animal data, which has shown that implanted cells can differentiate into multinucleated myocyte-like cells, resulting in improved global ventricular performance without the need for electromechanical coupling between donor cells and host cardiomyocytes (Murry, Field et al. 2005).
At this time, the clinical feasibility of this form of cell transfer in the management of ischemic heart disease has mostly been reported in multiple descriptive, pilot studies (Murry, Field et al. 2005). It has been shown that hundreds of millions of myoblasts can be grown from a small muscle biopsy under GMP conditions. Several uncontrolled studies have demonstrated modest improvements in ejection fraction and regional wall activity after skeletal myoblast transplantation (Herreros, Prosper et al. 2003; Menasche, Hagege et al. 2003; Siminiak, Kalawski et al. 2004). At least two small randomized trials using autologous bone marrow mesenchymal cells have also demonstrated improvements in cardiac performance, including increased myocardial fluorodeoxyglucose uptake, enhanced wall motion, a reduction in ventricular end-systolic and end-diastolic volumes, and a 14% net increase in ejection fraction, when compared with a saline-infused control group (Chen, Fang et al. 2004; Murry, Field et al. 2005).
The optimal cell type for this therapeutic application, however, remains to be defined (Elnakish, Hassan et al. 2012). At the same time, the mechanisms for the observed clinical benefits of cellular cardiomyoplasty remain unknown. Because cell survival after the procedure appears to be quite low, proponents have speculated that the improved myocardial indices may be secondary to other factors, such as increased angiogenesis, minimization of deleterious ventricular remodeling, and/or enhanced cytokine-mediated resident cell survival. Further studies are certainly needed in order to adequately assess the risks and benefits of cellular cardiomyoplasty. Also, whether or not the direct myocardial injection of donor cells may also be associated with tumors and/or an increased risk for malignant arrhythmias warrants further scrutiny.
Several products based on the principle of cellular cardiomyoplasty have been, or are currently being tested in controlled clinical trials, both in the United States and in Europe. One such therapy, marketed as MyoCell™ (Bioheart, Inc.) involves a collaboration of multiple centers and has entered phase III trials as a result of encouraging initial results (Haider, Lei et al. 2008). In this approach, autologous skeletal myoblasts are expanded ex vivo and supplied as a cell suspension to be delivered directly into the epicardium during coronary artery bypass grafting surgery. In the United States, a donor-derived bone marrow preparation, marketed as Prochymal™ (Osiris Therapeutics) is being evaluated in a phase I trial, with preliminary data suggesting that the product is safe when administered intravenously soon after myocardial infarction.
5.1.1. Congenital anomalies
Congenital heart disease is the leading cause of neonatal death from birth defects. Single ventricle anomalies are particularly threatening, often requiring multiple operations within the first few months of life in order to prevent irreversible congestive heart failure. A procedure commonly performed in these patients includes the separation between the pulmonary and systemic circulations by creating a cardiac total cavopulmonary connection using a biocompatible synthetic conduit (e.g., Dacron or Teflon). Unfortunately, these synthetic conduits are prone to thromboembolism and infection, and do not grow with the patient. As a consequence, many of these patients undergo risky re-operations to revise the conduit, in procedures associated with significant mortality and morbidity. A tissue engineered blood vessel conduit composed of autologous cells seeded on a biodegradable scaffold would avoid the many disadvantages associated with synthetic conduits. In both large animal models and humans, investigators have shown that tissue-engineered blood vessels can be made using a tubular scaffold composed of polyglycolic acid woven with either ε-caprolactone or L-lactide seeded with autologous bone marrow mononuclear cells for just 2 hours prior to implantation (Shin’oka, Matsumura et al. 2005). At least 25 children with congenital heart disease were followed for over 9 years after having received comparable tissue-engineered grafts, with encouraging results (Shin’oka, Imai et al. 2001; Hibino, Shin’oka et al. 2005; Hibino, McGillicuddy et al. 2010).
This early experience pointed to the feasibility and safety of clinical tissue engineering in pediatric cardiac surgery, but also uncovered the complication of graft stenosis secondary to neotissue formation in up to 20% of patients (figure 3) (Duncan and Breuer 2011). Fortunately, so far such complication has generally been amendable to percutaneous angioplasty. After further animal studies, the first U.S. patients were approved by the FDA to receive engineered vascular implants in a controlled trial starting in 2012. It remains to be determined whether the next generation of engineered blood vessel conduits will be associated with lower long-term morbidity after pediatric cardiac repair (Naito, Shinoka et al. 2011).
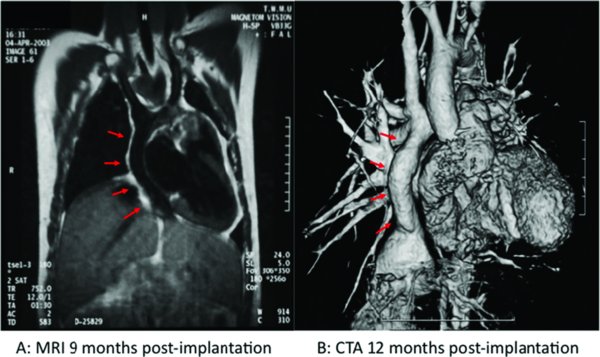
Growth potential of human engineered vascular grafts. A) Magnetic resonance image (MRI) 9 months following implantation. B) Three-dimensional computed tomography (CT) angiogram one year after implantation. Red arrows indicate location of the implant.
Anecdotal clinical experience with heart valves engineered from autologous endothelial progenitor cells has also started to be reported, with encouraging results (Cebotari, Lichtenberg et al. 2006). This methodology benefited from the presence of a small population of CD34 positive mononuclear hematopoietic progenitor cells in human peripheral blood capable of differentiating into the endothelial lineage in culture (Asahara, Murohara et al. 1997; Assmus, Schachinger et al. 2002), as this obviates the need for vascular procurement. Nevertheless, in addition to the endothelial lining, a mesenchymal population is typically needed to maintain the extracellular matrix and overall integrity of the valvular construct, for which MSCs have been explored. The optimum cell sources and scaffold –cell interactions remain to be established. Yet another significant and somewhat unique limitation of heart valve engineering stems from the complex and dynamic demands of the constantly moving three-dimensional environment, as well as its long term stability, particularly in pediatric applications. Still, in a separate study involving 11 patients, autologous endothelial progenitor cells isolated from a forearm vein were used to seed valve allografts with good long-term results at 10 years follow up (Dohmen, Lembcke et al. 2011). At the same time, given that most conventional acellular allografts tend to fail between 15 and 20 years after implantation, longer term follow up will be necessary in order to possibly establish the clinical validity of heart valves engineered with this methodology.
5.2. Neural repair
Parkinson's disease is a prevalent and debilitating neurodegenerative disorder caused by a selective loss of mesencephalic dopaminergic neurons within the substantia nigra. Affected patients have characteristic symptoms, including bradykinesia, resting tremors, muscle rigidity, and gait disturbance. Current medical therapy, including the exogenous administration of dopamine (levodopa), can be effective in many patients but in some is associated with numerous secondary motor complications.
Another type of simple cell transfer, namely the transplantation of human fetal ventral mesencephalic dopaminergic cells, has been studied for many years as a potential treatment for Parkinson's disease. Initial observational studies suggested prolonged survival of the transplanted cells, as well as substantial clinical neurological improvements in the absence of medication, with some recovery of the nigrostriatal pathway for more than a decade following the operation. Nevertheless, at least two randomized controlled trials comparing the effectiveness of human fetal cell transplantation with sham surgery demonstrated no significant differences between the two groups, except for some modest gains in a cohort of younger patients (Freed, Greene et al. 2001; Olanow, Goetz et al. 2003). Furthermore, both the practical and regulatory limitations of human fetal cell transplantation have precluded its widespread clinical application. Moreover, the transplantation of human fetal ventral mesencephalic dopaminergic cells has also been associated with the occurrence of adverse effects in the mid to long term, such as graft-induced dyskinesias (GIDs) stemming from graft-derived striatal serotonergic hyperinnervation (Politis, Oertel et al. 2011).
A number of investigators have shifted their focus from fetal cell transplantation to approaches that employ neural stem cells, which have a high capacity for self-renewal and could conceivably supply an abundant number of specialized neurons for the treatment of Parkinson's disease. One biotechnology company, NeuroGeneration, is seeking regulatory approval for phase II clinical trials of a strategy that includes neural stem cell-derived dopaminergic cells delivered into the affected striatal structures of patients with Parkinson's Disease. Other methodologies encompassing different cell sources/types and/or delivery methods continue to be pursued, though also have yet to be validated (Braak and Del Tredici 2008; Rossler, Boddeke et al. 2010). The use of neural and other stem cells in spinal cord repair is of course another area of even greater experimental activity, not within the scope of this review.
5.3. Bone repair
Different forms of bone loss can occur in a variety of pathological entities. Fairly established bone replacement methodologies include autologous and allogeneic bone grafts, demineralized bone, and numerous natural or synthetic bone substitutes, all still fraught with substantial limitations. Thus, in addition to growth factors and cytokines such as Bone Morphogenic Proteins (BMPs), the use of cell-based methods has been explored. Mesenchymal stem cells are natural candidates for bone replacement and indeed a number of anecdotal cases and small series of MSC-based tissue engineering to that end have been reported to date. Injectable suspensions of bone marrow MSCs have been used as a means to enhance bone healing in the treatment of congenital and acquired bone defects for decades (Salama and Weissman 1978; Jackson, Scheker et al. 1981; Healey, Zimmerman et al. 1990; Kitoh, Kitakoji et al. 2004; Hibi, Yamada et al. 2006; Hibi, Yamada et al. 2006). However, the first instance of bone repair using a three-dimensional cell-seeded construct, by Vacanti et al., took place only in the late 1990’s, on a patient with traumatic avulsion of a distal phalanx, in whom partial local bone formation was documented post-operatively (Vacanti, Bonassar et al. 2001). In this case, no stem cells were used, but rather osteoblasts obtained from a periosteal biopsy. Reports of different forms of bone repair with bone marrow MSC-seeded three-dimensional constructs have followed, typically in very small series or case reports (Ohgushi, Goldberg et al. 1989; Quarto, Mastrogiacomo et al. 2001; Warnke, Springer et al. 2004; Marcacci, Kon et al. 2007). Although variable degrees of osteoregeneration have been documented in these studies, the role of stem cell-based tissue engineering in bone repair remains to be properly defined. One additional interesting development, also still in its infancy, is the use of MSCs, for example procured from fetal liver, delivered prenatally to ameliorate genetic bone disorders, such as osteogenesis imperfecta (Horwitz, Prockop et al. 1999; Horwitz, Prockop et al. 2001; Guillot, Abass et al. 2008). Also here, while the results of the first clinical applications have been encouraging, much remains to be achieved before this principle, as all as the other MSC-based therapies discussed above, can be fully validated and broadly recommended.
5.4. Skeletal muscle repair
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder caused by a defect in the encoding for dystrophin, a muscle fiber stabilizing protein. The disease results in chronic injury to skeletal myocytes, leading to a vicious cycle of myocyte degradation and fibrosis. Most affected children are wheelchair-bound by early adolescence and, by early adulthood, most patients develop severe respiratory failure and cardiomyopathy. Despite significant advances in the understanding of the pathophysiology of DMD, its management continues to be largely supportive as no effective therapy yet exists for this disease.
One experimental approach to the treatment of DMD has been to enable relocalization and expression of normal functional dystrophin transcripts within the muscle through the transplantation of muscle precursor cells from normal donors, a procedure known as myoblast transfer therapy (MTT). This approach was originally shown to have promising results in animal models using mdx mice. Initial clinical experience showed evidence of dystrophin transcript expression in DMD patients by reverse-transcriptase polymerase chain reaction (Law, Goodwin et al. 1992). However, multiple clinical trials to date have not shown any objective benefit in DMD patients injected with donor myoblasts. In one of the earlier human studies, dystrophin-positive fibers comprised up to 36 percent of the injected muscles after 1 month (Gussoni, Pavlath et al. 1992). Nonetheless, such expression has generally been undetectable by 6 months post-injection. A subsequent study employing serial injections of normal myoblasts procured from unaffected relatives over a 6 month time period also did not show any clinical benefit (Mendell, Kissel et al. 1995).
These disappointing clinical results to date have likely been due to multiple factors, including poor cell survival, immune rejection, and limited cell distribution after injection. To our knowledge, there are no clinical trials of MTT currently ongoing. Still, the past few years have witnessed a renaissance in preclinical efforts to hopefully enable MTT eventually. These studies are attempting to address some of the shortcomings of the previous human trials, using enhanced immunosuppressive regimens and alternative stem/progenitor cell sources such as muscle-derived stem cells, among other approaches (Urish, Kanda et al. 2005).
5.5. Urologic repair
Complex urologic reconstructions of the bladder, ureter, and urethra have traditionally relied on the use of heterotopic autologous grafts such as stomach, intestine, or colon. None of these structures constitute ideal urologic replacements in that they lack urothelium and are associated with significant morbidity, including urolithiasis, metabolic disturbances, and malignant degeneration. Many investigators have long been evaluating tissue engineering approaches as potential means to overcome these common complications of urologic reconstruction, leading to some clinical experience.
Initial clinical applications of tissue engineering in urology focused on bladder augmentation (cystoplasty). This procedure is usually indicated for the management of high-pressure, low compliant bladders secondary to spina bifida, spinal cord injury, or urge incontinence. Although anti-cholinergic medication and intermittent bladder catheterization can be effective, surgical reconstruction with intestine remains as the standard in severe cases. To date, engineered bladders, referred to as Neo-Bladder AugmentTM (Tengion, East Norriton, PA), have been implanted into several children with myelomeningocele (MMC) and end-stage bladder disease, albeit only in a phase I trial (Atala, Bauer et al. 2006). Despite these early feasibility results, Tengion abandoned plans for phase II trials and further work in 2011, in large part due to the limited to no clinical efficacy and the occurrence of serious adverse events. The trial was also hampered by the lack of a control group (i.e., standard enterocystoplasty), as well as the absence of data on innervation, in part because of the underlying disease.
In contrast to bladder augmentation, one area of urologic tissue engineering that has received much attention in recent years is urinary diversion for bladder cancer (Basu, Jayo et al. 2012). Bladder cancer is one of the most common malignancies worldwide, with over 20,000 urinary diversions performed in the United States and Europe annually. The current standard for urinary diversion involves utilizing a segment of intestine to transport urine through a hole in the abdominal wall into a standard ostomy bag. As of early 2012, phase I clinical trials for Tengion's tissue engineered urinary conduit (Neo-Urinary ConduitTM) were ongoing at five U.S. centers in patients requiring urinary diversion following cystectomy for bladder cancer. Neo-Urinary ConduitTM carries urine from the ureters to the skin surface using a polylacetate glycolate scaffold seeded with autologous smooth muscle cells isolated from autologous abdominal fat.
Similar techniques have been used to implant other tissue-engineered urological structures in humans, including tubularized conduits for traumatic injury of the membranous urethra (Raya-Rivera, Esquiliano et al. 2011). Also here, further controlled analyses are needed to determine the value of tissue-engineered urethral replacements as an alternative to buccal mucosa or skin in patients requiring urethroplasty. Indeed, as it is the case in virtually all other reports involving clinical application of engineered grafts to date, the clinical experience with tissue engineering in urology lacks controlled trials, and none of the products are approved by the FDA as of this writing.
5.6. Airway reconstruction
Despite the relative rarity of surgical airway disease, engineered airway conduits have been of substantial interest for the treatment of a variety of airway ailments for many years now (Macchiarini, Walles et al. 2004; Kunisaki, Freedman et al. 2006; Macchiarini, Jungebluth et al. 2008; Lange, Fishman et al. 2011; Gray, Turner et al. 2012). Except for primary repairs, tracheal reconstructions remain frequently associated with suboptimal functional results and substantial morbidity and mortality. Although the trachea is a hollow conduit, its structure and biomechanical properties are in fact quite complex and demanding. Accordingly, the ideal engineered substitute needs be airtight, nonimmunogenic, noncollapsible, well-vascularized, well-epithelialized, and conducive to sustained chondrogenesis (Baiguera, D’Innocenzo et al. 2011).
The early clinical experience with tissue-engineered tracheas has been arguably promising. To date, a number of patients in Europe have undergone tracheobronchial replacement with a cellularized engineered construct, with interesting results (Macchiarini, Jungebluth et al. 2008; Baiguera, D’Innocenzo et al. 2011). Both decellularized airway and synthetic scaffolds have been used, usually seeded with autologous bronchial epithelial cells and bone marrow-derived MSCs and/or differentiated chondrocytes.
One limitation of the current approach in tracheal engineering has been the protracted time required to fabricate these constructs. In attempts to streamline the process, more recent techniques have incorporated the use of nano-composite polymer and growth factor-induced endogenous stem cell mobilization as means to enhance engraftment and remodeling in vivo (Jungebluth, Alici et al. 2011). At the same time, however, once more long-term follow-up, along with comparisons in controlled phase III trials will be essential before more widespread application of this technology can be justified.
6. Future perspectives
Tissue engineering remains in the early phase of its developmental curve. As evidenced in many examples discussed in this chapter and others, concrete clinical benefits from this technology have accrued in the past several years, albeit still at a much slower pace than what we can expect for the future, particularly as stem/progenitor cells become increasingly more studied in these applications. In addition to the therapeutic implications of the use of stem cells in tissue engineering, related studies can also lead to unique insights into more basic aspects of stem cell biology, which in turn may bring further, as yet unsuspected therapeutic developments. Although the widespread availability of “custom-made” tissues and organs has yet to become a reality, the field is both very young and very vibrant. Much remains to be learned and developed, not only by scientists and clinicians, but also entrepreneurs and regulatory agencies. Nevertheless, given its scientific premises, the potential magnitude of its impact to society, and what has been achieved thus far, it is reasonable to envision stem cell-based tissue engineering eventually reaching conventional clinical practice, from fetal medicine to geriatrics and the entire gamut in between.
References
Last revised August 28, 2012. Published December 10, 2012. This chapter should be cited as: Dionigi, B. and Fauza, D.O., Autologous Approaches to Tissue Engineering (December 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.90.1, https://www.stembook.org.