1. Introduction
This protocol outlines the techniques used to routinely passage hPSCs in the SKI Stem Cell Research Facility at Sloan-Kettering. We prefer to culture our cells on mouse embryo fibroblasts (MEFs) but occasionally grow the cells feeder free for particular applications, such as nucleofection, viral transduction or karyotyping.
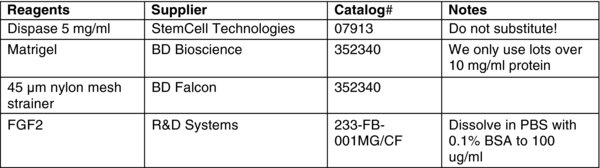
We use Dispase for all applications requiring colony passage including feeder-dependent and independent expansion as well as for cryopreservation.
2. Flowchart
2.1. Matrigel coating dishes
-
To expand cells feeder free, you must first prepare Matrigel-coated dishes. The preferred method for doing this is to remove a 1 ml aliquot (in a 50 ml conical stored at −20°C, see Materials) and place on ice overnight. In a pinch, you can warm it carefully in your hand, but make sure to place it on ice before it completely melts.
-
Add 19 ml of cold DMEM:F12 to the Matrigel. Sometimes, small pieces of polymerized Matrigel form. These do not interfere, but you can filter them out using a 100 μM strainer if desired (see Materials).
-
Add Matrigel to your surface.
-
Incubate in the hood for one hour.
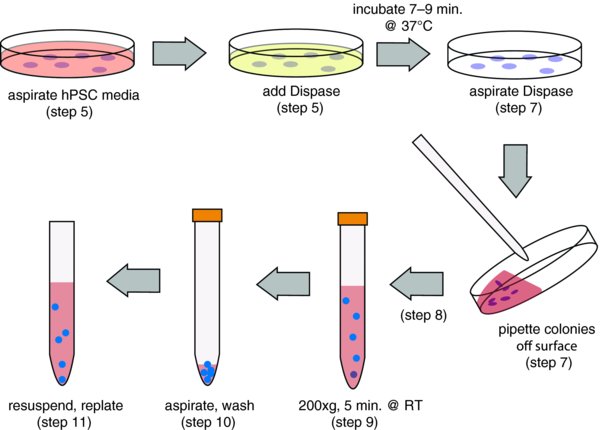
3. Passage of cells using Dispase
-
When cells are ready for passage, remove hPSC media and add the appropriate volume of 5 mg/ml Dispase.
-
Incubate for 7–9 minutes at 37°C in the incubator. After 7 minutes, check on the microscope to verify that the edges have started to detach. If they have not, return to the incubator for another minute and then check again.
-
Once they have started to peel, remove the Dispase and detach colonies from the cell surface with a small volume of hPSC media. Generally, this can be the same volume that you used for the Dispase (e.g., 2 ml for a 6 cm dish). You should be able to dislodge the colonies with relatively gentle pipetting. Try to keep the colonies as large as possible while removing them.
-
Add the detached colonies to an adequate volume of hPSC media, DMEM:F12 or even PBS to wash. Dispase is not inhibited with media, so dilution is required to inactivate the enzyme. I prefer to use 5 times the volume of dispase used per wash.
-
Spin at 200xg for 5 minutes at room temperature. Alternatively, you can just let the clusters settle with gravity for 5 minutes in the hood. This might help to eliminate single cells that are thought to contribute to karyotype problems in hPSC cultures.
-
Aspirate wash, carefully avoiding cell clusters. (Wash a second time if you are plating without feeders)
-
Resuspend in hPSC media. I typically resuspend in 12 ml of hESC media and plate 1 ml to a new dish (1:12), but this must be adjusted for each culture. Generally it is in the range of 1:6–1:20.
4. Troubleshooting
Use the Dispase specified in Materials: not all Dispases are the same. This protocol will only work for this preparation. We have used Worthington in the past too, but it is stronger and requires a second wash. When passaging cells without feeders, they are exquisitely sensitive to any residual enzyme left with the cells. Make sure you wash with a large volume and wash 2 times. If you are having trouble with the cells not attaching, you might even want to wash 3 times.
Keep in mind that as long as the colonies are still attached, you can wash the colonies by adding media/PBS to the dish and aspirating in place. This is much faster than spinning them down. At least one wash/spin should be done after you have detached colonies, however.
Dispase can not be inhibited by media so it is not necessary to use complex media to wash cells. Dilution is the only way to eliminate the enzyme.
5. Preplating to remove residual feeders
If you are moving your hPSCs from feeders to a feeder independent condition, you might want to preplate cells to remove feeders. Basically, you prepare your cells as you normally would be let them attach to gelatin-coated tissue culture treated plastic for one hour in the incubator. Under these conditions, the MEFs will adhere to the dish but the hPSCs will poorly attach to this plastic. After the hour incubation, remove the dish, pipette over the surface of the dish gently to dislodge the hPSCs, and transfer to a new Matrigel-coated dish leaving the attached fibroblasts behind.