1. Introduction
Individualization leads to severe cell death in human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). This phenomenon leads to difficulties in handling cells in applications such as passaging, cryopreservation and transfection, and the treatment with ROCK inhibitors has been found to effectively improve cell survival. However, enzymatic dissociation and ROCK inhibitor treatment could bring potential complication in cell maintenance. An enzyme-free dissociation method was developed to passage stem cells and achieve robust cell survival in dependent of drug treatment.
Here we describe how to propagate hPSCs as small aggregates using homemade EDTA/PBS solution. This method is suitable for feeder-free culture conditions, and has been used for StemPro, mTeSR and E8 media2. In companion with E8 medium and vitronectin (or synthetic surface), human pluripotent stem cells could be maintained in an enzyme-free, xeno-fee and chemically defined environment. For the simplicity, we used matrigel as the coating surface for cell culture in this protocol.
2. Protocol
-
Matrigel preparation.
-
Always store matrigel (BD Biosciences #354230) in non-antifreeze freezer.
-
One day before experiment put one bottle of matrigel on ice in 4°C cold room overnight.
-
Aliquot 2 mg of Matrigel into each pre-chilled 2 ml epitube, and immediately freeze. Matrigel concentration is usually 7∼10 mg/ml, check the concentration on the product sheet. 2 mg matrigel will be used to coat 2 6-well plates.
-
-
Plate coating.
-
Pour cold 12 ml of DMEM/F12 in conical tube, and use 1.5 ml to resuspend frozen matrigel with 5 ml pipet. Matrigel should be in freezer right before the experiment.
-
Rinse the matrigel tube again with the same media.
-
Mix the matrigel well, plate 1 ml in each well, and shake well to cover all the surface.
-
Leave the plate at room temperature or 37°C for at least 30 minutes. Or coat overnight at 4°C.
-
-
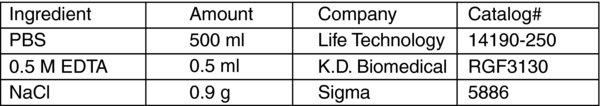
EDTA dissociation buffer
-
Add 500 ul 0.5 M EDTA and 0.9 g NaCl into 500 ml Calcium/Magnesium free PBS (Invitrogen # 14190).
-
Sterilize by filtration, and store at 4°C.
-
-
Dissociation with EDTA.
-
Have a matrigel plate that has sat at room temperature for at least half an hour, set aside.
-
Rinse wells to be split with 1 mL/well of warmed EDTA medium to wash away the Mg++ and Ca++ from the DF12.
-
Repeat step 2 to ensure all of the Mg and Ca is removed.
-
Add 1 mL/well EDTA medium for the third time, set plate aside and let sit 2–5 minutes (more than 2 minutes will result in very tiny colonies of only 3–5 cells).
-
While cells are sitting with the EDTA, label new plate and add 1.5–2 mL E8/well, let sit in hood until ready to use. If splitting more than one or two wells at a time you may want to have the new plates all ready with label and E8 before starting to EDTA the cells, because there isn't very much time to do that during the “incubation.”
-
After cells have sat with EDTA, aspirate EDTA and add 4 ml E8 with fast speed to disperse the cells as fast as possible. For the appropriate split ratio and wash the cells off, be careful not to break up the colonies too much–now the Mg++ and Ca++ in the E8/TeSR1 are needed to neutralize the EDTA.
-
Make sure the cell suspension is well mixed and plate desired cell amount per well. Discard any unused cell suspension.
-
Place in incubator, shake back and forth, side to side to distribute. Let sit overnight to ensure maximum attachment.
-
3. Materials
4. Troubleshooting
-
Cells come off the plate during EDTA/PBS treatment
-
Generally, we recommend to load EDTA/PBS gently into the plate, do not disturb the cells, so no centrifugation is needed in the process.
-
Alternatively, directly harvested with medium, and spin cells down by centrifugation.
-
-
Poor cell survival
-
Cells are too old or too confluent before passage. We usually passage cells every 3–4 days, and before the confluence reach beyond 80%.
-
If people feel comfortable with ROCK inhibitor treatment, 10 μM Y27632 could always be added as insurance,
-