1. Introduction
This protocol was developed in the Salk STEM Cell Core to enable researchers to consistently and reproducibly produce reprogrammed iPS cells, the initial idea came via word of mouth reports of its effectiveness to increase the efficiency of viral transduction. It has most commonly been used on retrovirus and lentivirus factors. Initial evaluation of this method showed 5–10× increase in transduction efficiency.
3. Protocol Steps
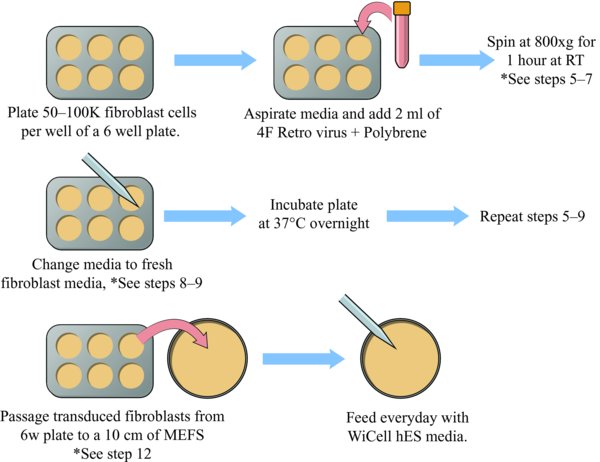
3.1. Prepare Plate For Spinfection
-
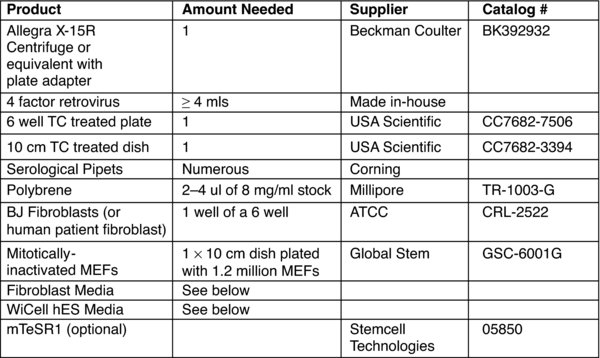
Prepare four factor (Oct4, Sox2, Klf4, c-myc) retrovirus to transduce fibroblasts. Note: This can be made months ahead of time and stored in the −80°C freezer.
-
Add Polybrene to the four factor retrovirus mixture. Note: Final concentration of Polybrene is 4–8 ug/ml.
-
Thaw retrovirus and mix all four factors together in equal amounts. Note: Use non-concentrated virus. Mix 0.5 ml of each retrovirus factor together for a total of 2 mls.
-
Plate 1 or more wells of a 6-well plate with an actively growing population of human fibroblasts. Note: The seeding density of the well to be transduced should be between 50,000 to a 100,000 cells per well (of a 6-well). *
3.2. Perform Spinfection
-
Aspirate the fibroblast media from the 6-well plate of fibroblasts.
-
Add 2 mls of warmed four factor retrovirus + Polybrene to each well to be reprogrammed.
-
Perform the spinfection at 800 xg for 1 hour at room temperature.
-
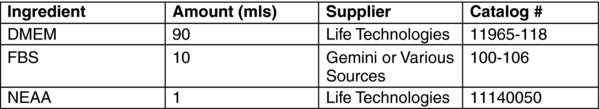
After the spin completes, aspirate the retrovirus media and add 2 mls fresh fibroblast media.
-
Allow the cells to recover for at least 6 hours (or overnight) in a 37°C incubator.
-
Repeat steps 5 through 7.
-
After the final spinfection, allow the cells to recover for 8–24 hours.
-
Passage 1 well of transduced fibroblasts onto 1×10 cm plate of mitotically inactivated-MEFs. Notes: Passage the fibroblasts using the enzyme Accutase and plating into fibroblast media. The MEFs are plated at a density of 1.2 million cells per 10 cm plate.
-
Alternatively, for a feeder free method, passage 1 well of transduced fibroblasts onto 3 matrigel coated wells. Note: Split the fibroblasts with accutase and plate in fibroblast media.**
-
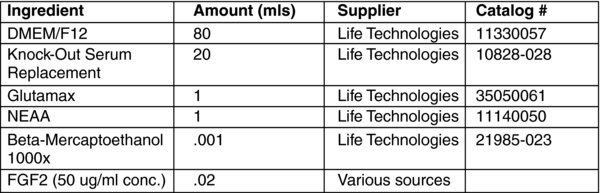
The next day, switch to feeding with WiCell hES media.
-
Feed plates every day.
Colonies will begin to appear within 3 to 5 weeks.
Be sure to handle virus and cell culture containing virus under the appropriate safety conditions. Consult with your local biosafety representative for details.
5. Notes
*Retroviral integration is dependent on actively dividing cells. Reprogramming efficiencies also have been shown to increase with increased proliferation rate of the starting cell population. For these reasons it is important to start reprogramming experiments with robust and actively dividing/growing cells. For human fibroblast we typically start with 50,000–100,000 cells per well (of 6 well). We typically transduce the fibroblast a day or two after passaging when the cells are sub-confluent, but are touching or nearly touching each other, and that ample open areas between the cells are still present. An extra well can be plated and counted at the time of infection if desired.
**We have had success using WiCell hES Media or mTeSR1. WiCell hES media (made in-house) is significantly less expensive than commercially purchased mTeSR1. We have had success plating transduced fibroblast onto matrigel coated wells and feeding with WiCell media (no MEFs, no conditioned media). Then, switching to mTeSR1 once colonies start to emerge and picking colonies directly onto matrigel/mTeSR1 conditions for expansion and characterization.
Alternatively (traditional approach) the transduced cells are passaged onto mitotically inactivated MEF feeder cells, and fed every day with WiCell (or similar) hES cell media. Make fresh media, store at 4 deg C and use within 2 weeks. Make fresh media as needed.