1. Introduction:
This protocol is used for general maintenance and passaging of hES and iPS cells grown on MEFs (Feeder-Dependent). It assumes that the cells are grown in a 6-well plate format.
3. Protocol:
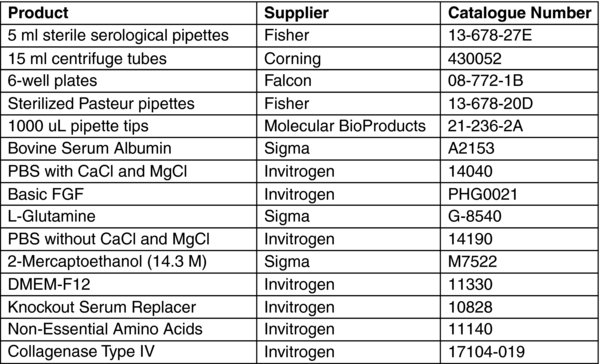
3.1. Media Preparation
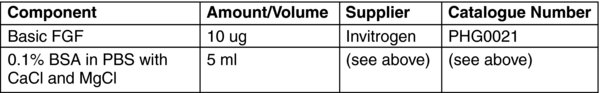
0.1% BSA in PBS (for Basic FGF Solution)
-
Dilute 250 mg of Bovine Serum Albumin (BSA) into 250 ml PBS with CaCl and MgCl.
-
Filter sterilize and aliquot into sterile 50 ml centrifuge tubes. Keep the working aliquot at 2–8°C, and freeze the other aliquots for up to 12 months at −20°C.
-
Briefly centrifuge the β-FGF vial.
-
Add 5 ml 0.1% BSA in PBS +Ca /+Mg to a 15 ml conical tube.
-
Remove 50 μl of the PBS with a 100 μl pipetman. Gently re-suspend the β-FGF, and add it into the 15 ml conical tube.
-
Remove 50 μl of the reconstituted β-FGF solution and return it to the vial to collect any residual protein. Return the solution back to the conical tube.
-
Aliquot the reconstituted β-FGF into 0.5 ml volumes in sterile 1.5 ml microcentrifuge tubes using a 1.0 ml pipetman.
-
Store aliquots in the −80°C freezer for up to 12 months.
-
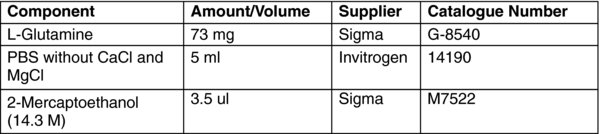
Dilute 73 mg L-Glutamine in 5 ml of PBS without CaCl and MgCl.
-
In the chemical fume hood, add 3.5 μl of 2-Mercaptoethanol and mix well.
-
Use the L-glutamine solution immediately, and aspirate any excess solution into the liquid waste container.
-
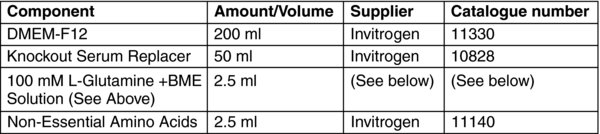
To make 250 ml of Stem Cell Culture Medium, combine the components from the above table, filter sterilize, store at 2–8°C, use for up to 14 days.
-
Weigh 50 mg of Collagenase Type IV powder into a weigh boat and transfer to conical tube, rinsing weigh vessel if necessary. Collagenase can also be weighed directly into tarred conical tube.
-
Transfer 50 ml of slightly warmed (room temperature) DMEM/F-12 medium into the conical tube, cap and invert to mix until dissolved.
-
Filter sterilize, store at 2–8°C, use for up to 14 days.
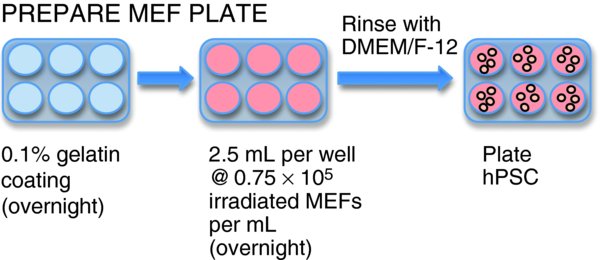
Prepare MEF Plate
-
Plate 1 ml/well sterile 0.1% gelatin into each well of a 6 well plate and allow to incubate at 37°C for a minimum of 1 hour (preferably overnight).
-
Aspirate excess gelatin and plate 2.5 mls of inactivated MEFs at a density of 0.75 × 10e5 cells/ml into each well of a 6 well plate.
-
Give the plate a gentle shake back and forth, side to side to help distribute the cells evenly, and allow to rest overnight at 37°C before using.
-
Immediately prior to use, aspirate medium, rinse plate with sterile DMEM-F12 Medium, aspirate and add 1 ml per well Stem Cell Culture Medium to the plate. Label plate appropriately with cell line and passage number, and any other required information.
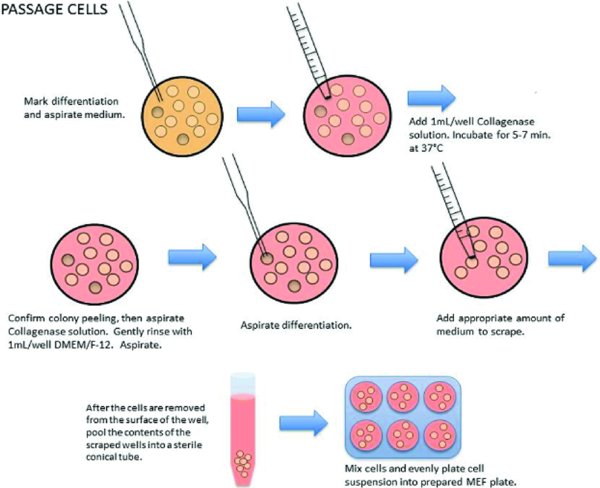
Passage the PSCs
-
Remove plate to be passaged from incubator and examine under microscope
-
Mark areas of differentiation on the wells to be split using the microscope objective marker, and determine split ratio.
-
In the biosafety cabinet, aspirate the spent medium from the wells to be passaged with a Pasteur pipette. At least one well of cells should be left and used as a backup to protect against problems with the split that would otherwise jeopardize the culture (contamination, etc.).
-
Add 1 ml room temperature Collagenase Solution to each well to be passaged. Incubate for 5–7 minutes at 37°C.
-
To confirm appropriate incubation time, view the colonies under the microscope. Look for the perimeter of the colony to appear highlighted or just slightly folded back. The colonies will not be coming completely off the plate.
-
-
Aspirate the Collagenase solution with a Pasteur pipette. Remove the collagenase carefully without disturbing the attached cell layer.
-
Gently add 1 ml of warmed DMEM/F-12 to each well with a 5 ml pipette, being careful not to remove the cells from the plate. Aspirate off the medium. NOTE: If cells wash off the plate, you may collect, spin, aspirate the supernatant and resuspend in appropriate volume of Stem Cell Medium.
-
If removing differentiation by suction, ensure the pipette tip is intact (free of chips) and carefully press the pipette tip to the marked area and suction off the differentiated cells. Confirm the complete removal under the microscope.
-
Add 2 ml of Stem Cell Culture Medium to each well.
-
Using a sterile 5 ml pipette, bring up medium into the pipette. Hold the pipette perpendicular to the plate and gently scrape the surface of the plate while simultaneously expelling medium. Repeat if necessary. NOTE: Minimize bubbles by scraping and pipetting gently.
-
Repeat until ∼90% of cells are rinsed from the plate.
-
After the cells are removed from the surface of the well, pool the contents of the scraped wells into a sterile conical tube.
-
Pipette cells up and down gently a few times in the conical tube to further break-up cell colonies if needed. Pipette carefully to reduce foaming.
-
Take up 1–2 ml Stem Cell Culture Medium in a 5 ml pipette and add it to the first well to wash and collect residual cells. Take up the medium and transfer it into each subsequent well to collect cells.
-
Transfer the Stem Cell Culture Medium wash to the conical tube containing the cells.
-
Place the appropriate amount of cell/media mixture into each well of the previously prepared MEF plate to achieve the desired split ratio. For example: for a 1 : 4 split, dilute cell pool to 4 mls/well collected (2 wells collected would dilute to 8 mls cell suspension total) and plate 1 ml of diluted cell pool/well.
-
Return the plate to the incubator after plating the cells. Move the plate in several quick, short, back and-forth and side-to-side motions to further disperse cells across the surface of the wells. NOTE: While cells are attaching, try to limit opening and closing the incubator doors, and if you need to access the incubator, open and close the doors carefully. This will prevent disturbing the even distribution of cells to the surface of the well.
-
Incubate cells overnight to allow colonies to attach.
-
Refresh with 2 ml/well Stem Cell Culture Medium daily until ready to passage or harvest.
Last revised March 28, 2012. Published June 10, 2012. This chapter should be cited as: WiCell, Feeder dependent (MEF) culture protocol - collagenase passaging (June 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.62.1, https://www.stembook.org.