1. Introduction
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), are known to be vulnerable to apoptosis upon various technical manipulation, such as single cell dissociation, freezing and thawing, etc., which hinder their use for clonal isolation in gene transfer, differentiation and FACS cell sorting.
However, Y-27632, a selective inhibitor of p160-Rho-associated coiled-coil kinase (ROCK) was found to be an effective inhibitor of apoptosis and enhanced survival of hPSCs upon single cell dissociation.
Here we describe how to propagate hPSCs in single cell dissociation using
Accutase, a ready to use cell detachment solution of proteolytic and collagenolytic enzymes and a direct replacement for trypsin solution.
2. Protocol
Preparation of MEF feeder plate
Coat culture dish with sterile 0.1% gelatin solution for about 5 min at room temperature.
Thaw frozen MEF vial at 37°C water bath quickly.
Transfer cells into 15 ml conical tube and add fibroblast media (DMEM + 10% FBS).
Wash cells via spinning at 1500 rpm, 5 min.
Plate MEF at a density of 1.5–2.0 × 104/cm2.
Let MEF settle down at least 8 hours before use.
(Overnight is best and MEF can be used within 2 days)
Splitting hPSCs with Accutase
Replace media for MEF dish with hES media.
Remove differentiated hPSCs colonies under microscope (optional).
Aspirate the media of hPSCs and add appropriate amount of Accutase (ex. 2 ml in 60 mm dish).
Incubate cells at 37°C incubator for 20 min until cells are in single cell suspension.
Harvest cells into conical tube and add hES media for wash.
Cell strainer with 40 μm-pore can be used to remove debris and cell clumps (optional).
Centrifuge cells for 5 min at 1000 rpm in hESC media to remove any remaining Accutse solution.
Resuspend cells with hES media and plate cells onto MEF feeder cells with density of 1.0–2.0 × 104 cells/cm2.
Add Y-27632 with a final concentration of 10 μM.
Incubate cells at 37°C incubator for the growth and feed with hES media everyday.
3. Materials
Cell Materials

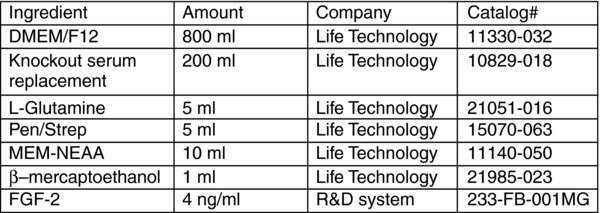
hES media (1000 ml)
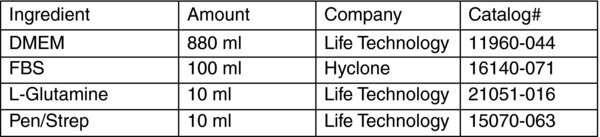
DMEM with 10% FBS (1000 ml)
Reagents
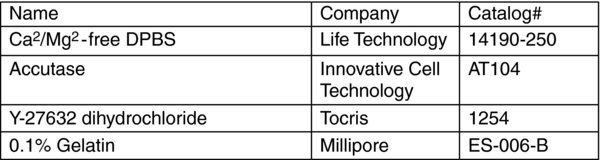
STUFF
4. Troubleshooting
Cells are still in clump with Accutase treatment
Remove clumps with cell strainer
Incubate cells enough time
Check Accutase
No colonies attached/survived
Wait until single cells form visible colonies for about a couple of days
Check Y-27632