1. Introduction
This protocol was developed as an efficient and consistent method to produce functional retroviral reprogramming factors. It avoids the need for concentrating virus via ultracentrifugation.
2. Flow Chart
3. Protocol Steps
3.1. Prepare 293T Cells
Grow 293T cells in a T175 flask. Note: A least 1 T175 flask per factor will be needed, so you must have at least 4 flasks. Each T175 should be fed with 32 mls of 293T Media.
Cells should be ∼85% confluent. Note: Cells are normally ready about 2 days after a 1:5 split.
3.2. Transfection of 293T Cells
3. Aspirate 293T Media from each 293T flask.
4. Feed each flask with 16 mls of fresh 293T Media. Tip: Label each flask with which viral factor it will receive (Oct4, Sox2, klf4, or c-myc).*
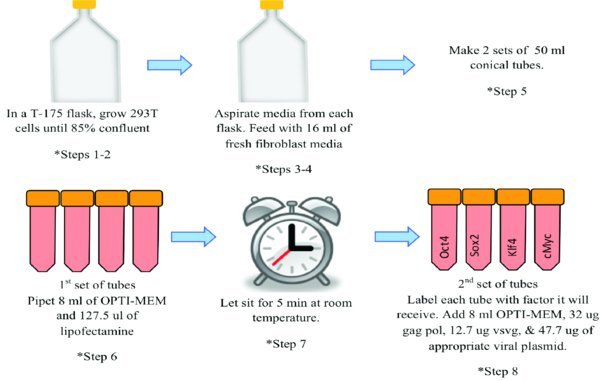
5. Make 2 sets of 4×50 ml conical tubes.
6. For the first set of 4×50 ml conical tubes: Give each tube 8 mls of OPTI-MEM media and 127.3 ul of Lipofectamine 2000.
7. Flick each 50 ml conical tube and let sit for 5 minutes at room temperature.
8. For the second set of 4×50 ml conical tubes: Label each tube with a different viral factor (Oct4, Sox2, klf4, or c-myc). Each tube gets 8 mls of OPTI-MEM, 32ug of gag pol, 12.7 ug of vsvg, and 47.7ug of appropriate viral plasmid.
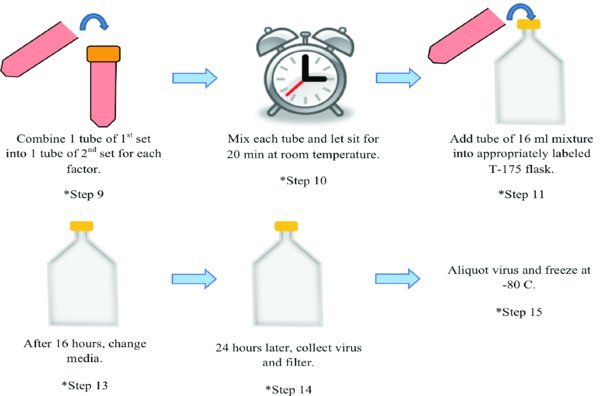
9. Combine one tube of the first set (OPTI-MEM + Lipofectamine 2000) with 1 tube of the second set (OPTI-MEM + gag pol + vsvg + viral plasmid) for each factor. Each tube will now have 16 mls total in it.
10. Mix each tube and let sit for 20 minutes at room temperature.
11. Add the appropriate tube of 16 ml mixture to its correspondingly labeled T175 flask.
12. Place in 37∘C incubator.
13. After 16 hours, change media and replace with 32 mls fresh 293T Media. Note: Remember to wear proper personal protective equipment. Some active virus might be present after 16 hours.
14. 24 hours later, collect the 32 mls of 293T Media from each T175 flask and filter. This media is now virus rich.**
15. Aliquot virus and freeze at −80∘C freezer.***
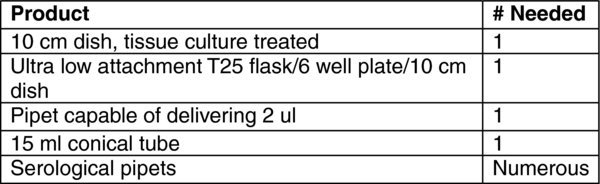
4. Materials
5. 293T Medium
6. Notes
*Be very gentle when feeding 293T cells. They will detach from tissue culture treated plastic very easily. We found that culturing 293T cells in a T175 flask allowed us to produce a large amount of virus in a single vessel and to feed 293T cells more gently.
**When using our spinfection protocol there is no need to concentrate the virus.
***In general, we like to combine the four factors together and aliquot into 4 mls. Each aliquot is then ready to reprogram one well of a 6 well plate when using our spinfection protocol. Virus can be stored at −80∘C for at least 6 months. Avoid repeated freeze thaw cycles with the virus.
****There is lot-to-lot variation with FBS that contributes to the overall efficiency of virus production using this protocol. It is advisable to screen multiple lots for efficiency of virus production. If lot screening is not an option, then HyClone sourced FBS is typically a safe bet.