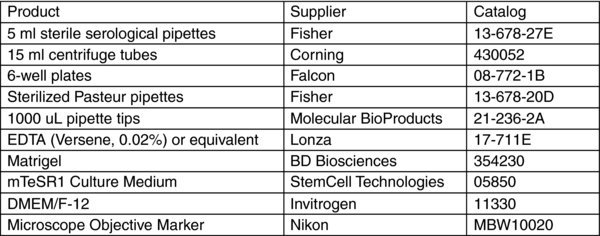
1. Introduction
This protocol is used for general maintenance and passaging of hES and iPS cells in a feeder-independent culture system such as mTeSR1/Matrigel. It assumes that the cells are grown in a 6-well plate format.
2. Flow Chart
3. Protocol Steps:
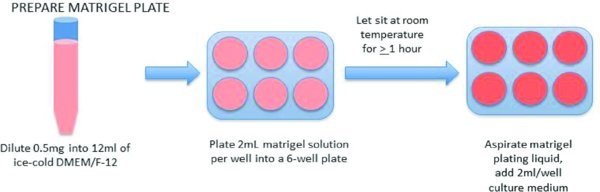
3.1. Prepare Matrigel Plate
Place a sterile 15 ml conical tube and a cold, sterile bottle of DMEM/F-12 into the sterile biosafety cabinet.
Remove one Matrigel™ aliquot (0.5 mg) from the freezer, and in the biosafety cabinet, immediately add 1 ml of cold DMEM/F-12 to the aliquot.
Gently pipette up and down to thaw and dissolve the Matrigel™. Immediately transfer it to the 15 ml conical tube.
Add an additional 11 ml of ice cold DMEM/F-12 to the 15 ml conical. Immediately plate 2 ml/well. This will be enough for 1 full 6 well plate.
Allow to set one hour at room temperature before use. If any portion of the well dries out, do not use the well.
If the plates will not be used the day they are made, either store in a 37°C incubator OR wrap the plate in parafilm, and store it at 2–8°C. Both storage techniques require using the plates within 7–10 days after preparation. Plates stored at 2–8°C must sit at room temperature for ≥1 hour prior to using. Plates stored in a 37°C incubator may be used immediately.
Immediately before passaging cell, aspirate excess Matrigel solution from plate, and add 2 ml fresh TeSR medium/well.
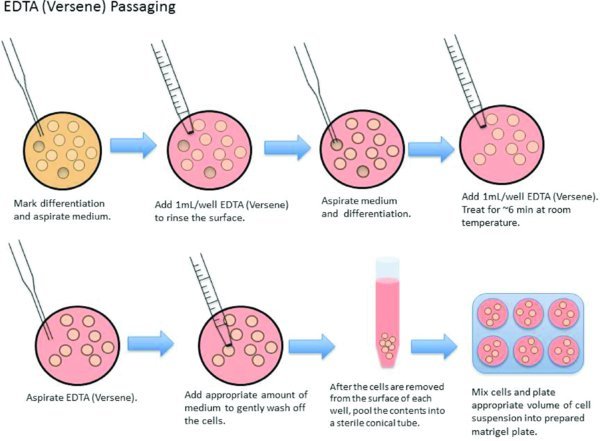
3.2. Passage PSCs Using EDTA (Versene)
8. Mark areas of differentiation on the well to be split using the microscope objective marker.
9. Examine plate and determine passaging ratio.
10. Aspirate the spent medium from the wells to be passaged with a Pasteur pipette. At least one well of cells should be left and used as a backup to protect against problems with the split that would otherwise jeopardize the culture (contamination, etc.)
11. Rinse each well with 1 ml room temperature EDTA (Versene). Aspirate EDTA and previously marked areas of differentiation at this time.
12. Add 1 ml room temperature EDTA to each well.
13. Incubate with EDTA for 5–7 minutes at room temperature.
14. While treating with EDTA, determine volume of cells to add to each new well by dividing total anticipated volume of collected cell suspension by the split ratio.
15. Aspirate the EDTA with a Pasteur pipette carefully without disturbing the attached cell layer.
16. Add desired amount of TeSR medium to each well for collecting cells (usually 2–3 ml/well). Do not work with more than 3 ml/well (overfilling wells may lead to contamination).
17. Holding a 5 ml pipette at a 90 degree angle, expel medium back and forth across the well to detach cells. You should be able to easily remove cells without touching plate (no scraping required).
18. After the pluripotent stem cells are removed from the surface of the well, pool the contents of the scraped wells into a sterile conical tube.
19. Gently re-suspend the cells using a 5 ml pipette.
20. Add appropriate volume of cell suspension (determined in step 14) to each well of the new plate.
21. Return the plate to the incubator after plating the cells. Move the plate in several quick, short, back and-forth and side-to-side motions to further disperse cells across the surface of the wells.
22. While cells are attaching, try to limit opening and closing the incubator doors, and if you need to access the incubator, open and close the doors carefully. This will prevent disturbing the even distribution of cells to the surface of the well.
23. Incubate cells overnight to allow colonies to attach.
24. Refresh Culture Medium daily with 2 ml/well until ready to passage or harvest.