Adult stem cells posses the ability to undergo both self-renewal and differentiation in multiple lineages. A recent body of work has utilised exogenous mesenchymal stem cells from the bone marrow compartment to attenuate lung injury. Initial studies suggested that bone marrow-derived stem cells (BMSCs) could repair damaged tissue by differentiating into epithelial cells in disparate sites. However this has been challenged and is now felt to be of limited clinical significance. What is clearer is that in chronic lung injury these cells are activated in response to tissue damage, migrate to the site of injury and contribute to both structural and functional repair. They are relatively non-immunogenic allowing them to be expanded and engineered ex vivo and re-introduced without immunomodulation. In acute lung injury BMSCs have been shown to reduce the pulmonary inflammatory response via a number of mechanisms to cause down-regulation of pro-inflammatory cytokines and a reduction in pathological lung damage. This chapter examines the role of exogenous bone marrow-derived cells, and in particular mesenchymal stem cells in both repair of chronic lung disease and acute lung injury, and their suitability as vectors for gene therapy.
1. Introduction
Three respiratory diseases, one acute and two chronic, stand out as lacking successful therapies and have been a focus for cell therapy studies. In an acute setting acute lung injury (ALI) is a major cause of respiratory failure. It is a significant cause of morbidity and mortality and contributes to both prolonged mechanical ventilation and increased in-patient stay. The mortality rate for ALI and the resulting acute respiratory distress syndrome (ARDS) is 40–50%, it accounts for greater than 3.5 million hospital days (Rubenfeld et al., (2005)) and only low tidal volume ventilation has been shown to reduce mortality ((Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury et al., 2000)). When considering chronic lung diseases lung cancer makes up 15% of all cancer diagnoses and is the most common cause of cancer death (Jemal et al., (2008)). The overall mortality rate from lung cancer is in the region of 80% (National Institute of Clinical Excellence (National Institute of Clinical Excellence., 2005)) and unless diagnosed at an early stage successful treatment options are limited. Interstitial lung disease is characterised by progressive lung fibrosis and debilitating breathlessness and is poorly responsive to current medical therapies with the only real treatment option being lung transplantation. Idiopathic pulmonary fibrosis (IPF) is usually fatal with only a 30% five-year survival (Perez et al., (2003)).
Stem cells are broadly classified into embryonic and adult stem cells. Embryonic stem cells are derived from the blastocyst and have the ability to produce progeny of all cell lineages (endoderm, mesoderm and ectoderm), a characteristic known as pluripotency. In contrast adult stem cells are lineage restricted and found in discrete niches within adult tissues where they divide infrequently. Here they repair damaged tissue by replacing specialised cells. Because of their restricted lineage adult stem cells were thought either multipotent, with the ability to differentiate into a limited range of cells or unipotent, with the ability to produce only one cell type.
The best studied of all adult stem cells are those of the bone marrow. Bone marrow derived stem cells (BMSCs) are either haematopoietic (HSCs), which produce the progenitors of mature blood cells or mesenchymal (BM-MSCs). BM-MSCs show both unlimited self renewal and the ability to differentiate into multiple lineages including fat, cartilage and bone. They have the ability to migrate to sites of injury, inflammation and to neoplastic masses and can affect tissue microenvironment via the secretion of soluble factors. These characteristics make them potential candidates for the treatment of autoimmune and vascular diseases as well as efficient vectors for the delivery of treatment to specific sites. This chapter focuses largely on BM-MSCs and their role in lung disease but does not cover the fast expanding literature on the role of endogenous stem cells in lung tissue repair.
2. Lung regeneration
Although initially thought to have a restricted cell lineage, studies undertaken at the start of this decade suggested that BMSCs may be capable of undergoing differentiation to produce progeny of other lineages (Jiang et al., (2002); Anjos-Afonso et al., (2004)) a characteristic known as plasticity. One of the seminal studies involved female mice with lethally irradiated bone marrow receiving a single HSC from a male donor. Using techniques of both immunohistochemistry and Y chromosome detection, cell engraftment was demonstrated in both the donor bone marrow cell lineages and also epithelial organs including the lung, which showed engraftment making up to 20% of the lung parenchyma (Krause et al., (2001)).
The concept of donor bone marrow stem cells engrafting in recipient lungs was reproduced in humans by means of sex-mismatched transplant patients. One study looked at female recipients of male HSCs and showed significant engraftment of donor-derived cells within the recipient lung parenchyma (Suratt et al., (2003)) whilst another showed similar results in male recipients of a female lung allograft (Kleeberger et al., (2003)). This study additionally demonstrated that engraftment levels at sites of chronic lung injury were significantly higher leading to suggestions that bone marrow stem cells were actively recruited by injured lung to aid repair.
To investigate this further, numerous studies have been performed where labelled bone marrow donor cells are infused into recipient mice. Cell labelling was either phenotypic [by means of green fluorescent protein (GFP) or β-galactosidase] or genotypic (Y chromosome) and the proportion of donor-derived cells measured. All forms of bone marrow cells have been studied including single bone marrow cells (Wagers et al., (2002)), MSCs (Kotton et al., (2001); Ortiz et al., (2003); Rojas et al., (2005)), HSCs (Aliotta et al., (2006)) and multipotent adult progenitor cells (Jiang et al., (2002)). A variety of experimental conditions have also been used to induce lung injury including: bleomycin (Kotton et al., (2001); Epperly et al., (2003); Hashimoto et al., (2004)), elastase (Ishizawa et al., (2004)), lipopolysaccharide (Yamada et al., (2004)), paracetamol (Direkze et al., (2003)) and radiation (Theise et al., (2002); Anjos-Afonso et al., (2004); Aliotta et al., (2006)). The results of these studies, however, have been conflicting with some showing no contribution of adult bone marrow stem cells to lung epithelial cell compartment whilst others showed significant engraftment as other stromal cell types.
The current consensus is that the initial differences in results were due in part to an overestimation by the techniques used to detect engraftment (Chang et al., (2005); Kotton et al., (2005); particularly immunohistochemical and fluorescence) and also due to the inconsistencies of study design and differences in donor cells. The more recent studies suggest that whilst there is likely to be some engraftment of BMSCs as lung tissue it is probably at much lower levels than previously suggested (Krause, (2005); Weiss et al., (2006)) and is unlikely to be of use in a clinical setting.
3. Lung injury
3.1. Chronic lung injury
Whilst there may be uncertainty over the clinical significance of the ability of BMSCs to engraft in the lung there is more agreement over the contribution of BM-MSCs to tissue healing and fibrosis.
3.1.1. Bone marrow cell mobilisation in response to injury
Initially tissue healing was thought to involve the recruitment of fibroblasts from neighbouring connective tissue but more recently it has been suggested that blood-borne fibrocytes are attracted to the area of damage in response to specific inflammatory cytokines (Bucala et al., (1994); Abe et al., (2001); Schmidt et al., (2003); Phillips et al., (2004)). Fibrocytes are pleiotropic and contribute to lung fibrosis via mechanisms including recruitment and activation of T cells that may be involved in early fibrosis, promotion of angiogenesis and collagen production via cytokine production (Strieter et al., (2007)). Their presence in bone marrow has been shown in a number of injury models. Pulmonary fibrosis induced by endotracheal bleomycin showed that 80% of type 1 collagen-expressing cells at the site of fibrosis were of bone marrow origin (Hashimoto et al., (2004); Ishii et al., (2005)) with similar results being demonstrated in radiation induced lung injury (Epperly et al., (2003)).
Interestingly although the bone marrow is a source of fibrocytes, it also appears to protect tissues using other cell types. A study using the bleomycin induced lung injury model showed murine BM-MSCs home to areas of injury, adopt an epithelial-like phenotype, and reduce inflammation and collagen deposition in lung tissue (Ortiz et al., (2003)). Similarly an animal model of emphysema caused by the administration of intranasal elastase demonstrated an improvement in outcome following the administration of bone marrow-derived cells that had been augmented by G-CSF (granulocyte colony-stimulating factor; Ishizawa et al., (2004)), Finally, inducing myelosuppression with busulphan increased the severity of the lung fibrosis and resulted in a higher mortality rate, a phenomenon that was reversed by BM-MSC infusion (Rojas et al., (2005)). These studies suggest that endogenous bone marrow is important for recovery from lung injury and that this can be augmented by exogenous BM-MSC administration.
3.1.2. Migration to site of injury
If tissue injury results in the mobilisation of BM-MSCs then the body has to provide a mechanism by which these cells are directed to areas of need. It is possible this occurs in a similar way to that by which leukocytes are recruited to areas of inflammation. HSCs have been clearly demonstrated to depend on the chemokine CXCL12 [stromal derived factor-1 (SDF-1)] and its receptor CXCR4 (Peled et al., (1999); Chute (2006)) whilst those involved in BM-MSC trafficking are less well defined. Numerous authors have tried to provide a comprehensive description of both the chemokines and growth factors responsible for BM-MSC migration and their related receptors (Honczarenko et al., (2006); Ponte et al., (2007); Ringe et al., (2007)) but there is still no clear agreement. However, the general consensus is that there are likely to be a variety of substances and receptors required for homing (Chamberlain et al., (2007)) and a combination of factors may be needed for optimal effects (Ozaki et al., (2007)).
A number of chemokine receptors contribute to fibrocyte migration in vitro including CC chemokine receptor 3 (CCR3), CCR5, CCR7 and CXCR4 (Abe et al., (2001); Phillips et al., (2004)). CXCR4 and CCR2 seem to have an important role in fibrocyte recruitment to the lung in murine models whilst CCR7 seems to have a role in renal recruitment (Moore et al., (2006); Sakai et al., (2006)). Fibrocytes have been shown to migrate to the lung in response to bleomycin induced lung injury resulting in an increased level of fibrosis, an effect reduced by CXCL12 inhibition (Phillips et al., (2004); see Figure 1). Both CXCR4 expressing cells and CXCL12 are present in higher levels in lung tissue samples from patients with idiopathic pulmonary fibrosis, and intratracheal installation of bleomycin in a murine model results in an increase in these levels in bronchoalveolar lavage samples, with a concomitant reduction in CXCR4 levels in the bone marrow. The induction of BM-MSC mobilisation was blocked by a CXCR4 antagonist with a corresponding reduction in fibrosis (Xu et al., (2007)). Whilst other chemokine/receptor combinations have been elucidated in mouse models, as mentioned above, their importance in relation to human models is unknown as human and murine fibrocytes show variations in their receptor expression.
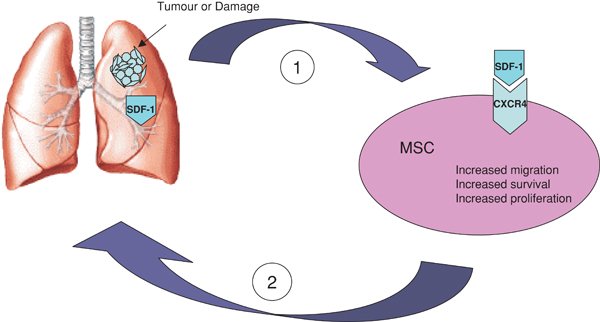
Figure 1. Schematic representation of selective migration of MSCs to areas of tissue injury and tumours where they contribute to tissue repair and tumour-associated stroma formation. (1) Lung injury stimulates MSC activation via interaction of SDF-1 with its receptor CXCR4. Acting in an autocrine fashion SDF-1 causes increased migration, survival and proliferation of MSCs. (2) Activated MSCs migrate to areas of injury and tumour in response to SDF-1.
It is possible that these pathways are also important in bone marrow recruitment to tumours. Small cell lung cancer (SCLC) tumour cells have been shown to express high levels of CXCR4 receptors and respond to the presence of CXCL12 activating an integrin signalling pathway. This pathway causes tumour cell invasion into the extracellular matrix and adhesion to marrow stromal cells conferring protection against chemotherapy induced apoptosis (Hartmann et al., (2004); Hartmann et al., (2005)), an effect that is inhibited by the presence of a specific CXCR4 antagonist (Burger et al., (2003)). Tumour cells from non-small cell lung cancer (NSCLC) also express high levels of functioning CXCR4 receptors that seem to be related to their metastatic potential (Su et al., (2005)). In a mouse model the common metastatic sites (brain, liver and adrenal glands) have higher concentrations of CXCL12 and the administration of an anti-CXCL12 antibody attenuated organ metastases (Phillips et al., (2003)).
3.1.3. Contribution to tissue stroma
Tissue stroma consists of both myofibroblasts and fibroblasts which produce collagen and other components of the extracellular matrix. They interact with epithelial and connective tissue cells and control angiogenesis (De Wever and Mareel, (2003); Desmouliere et al., (2004); Direkze and Alison, (2006)). Myofibroblasts secrete growth factors and proteolytic enzymes that effect tumour invasion and progression (Orimo et al., (2005)) and increased stroma and myofibroblast numbers have been related to a poorer prognosis (Cardone et al., (1997); Barth et al., (2002)). In the case of malignancy the tumour stroma not only provides support but it is essential for the behaviour of the tumour cells (Bhowmick et al., (2004)). The proliferative activity of stromal fibroblasts has been correlated with lymph node and distant organ involvement of breast carcinoma (Hasebe et al., (2000)) and activation of stromal cells by irradiation results in an increased invasiveness of pancreatic cancer cells, in vitro (Ohuchida et al., (2004)).
Bone marrow cells contribute to tumour stroma formation in the form of myofibroblast and fibroblast production. A number of xenograft tumour models have studied the fate of labelled bone marrow derived myofibroblasts and endothelial cells and shown that up to 25% of tumour myofibroblasts were of bone marrow origin (Ishii et al., (2003); Direkze et al., (2004)). The level of contribution depends on both tumour cell type and the site of implantation (Sangai et al., (2005)) but they do seem to be functional as shown by the production of collagen (Hasebe et al., (2000)).
One of the essential mechanisms for malignancy is tumour neovasculogenesis and bone marrow-derived precursor cells have been shown to contribute to this process (Lyden et al., (2001)). Labelled Sca1+ bone marrow cells incorporate as endothelial like cells into the periphery of a glioma (Anderson et al., (2005)) and when these cells are transduced with a suicide gene (HSV-tk) they induce an increase in apoptosis and reduction in tumour size (Ferrari et al., (2003)). This ability to engraft has been shown by BMSCs in a variety of other tumours including Kaposi's sarcoma (Khakoo et al., (2006)), colorectal cancer (Menon et al., (2007)), breast metastases (Studeny et al., (2004)) and melanoma metastases (Xin et al., (2007); Studeny et al., (2002)).
3.2. Summary
Bone marrow derived stem cells show unlimited self-renewal and plasticity
Initial studies suggesting they engraft at sites of tissue injury have been recently challenged and felt to be of limited clinical relevance
They are mobilised in response to injury and trigger fibrocyte production
Fibrocytes are directed to sites of damage in response to a number of chemokines and their receptors
At sites of injury BMSCs contribute both to tumour stroma formation in the form of myofibroblasts and fibroblasts which secrete extracellular matrix and to tumour neovasculogenesis
4. Acute lung injury
ALI is characterised by diffuse alveolar damage and an increased permeability of the alveolar-capillary membrane. Plasma proteins and fluid leak into the alveolar space causing pulmonary oedema and impaired gas exchange. Neutrophils and macrophages accumulate in the interstitium and pro-inflammatory cytokines are released into the lungs. This process is most commonly triggered by severe sepsis (Rubenfeld et al., (2005)).
Murine studies using an endotoxin triggered model of ALI have shown that administration of BM-MSCs soon after injury results in reduced vascular permeability, alveolar oedema and pulmonary haemorrhage. There is a reduction in the levels of pro-inflammatory cytokines (INF-γ, IL-1β, IL-6 and macrophage inflammatory protein 1α) and additional up-regulation of anti-inflammatory cytokines (IL-10), a response thought to be mediated by both soluble factors and direct cell-cell contact (Xu et al., (2007)). This occurs despite low levels of stem cell engraftment within the lung parenchyma and confers a survival benefit (Gupta et al., (2007)).
Although the precise mechanisms by which BM-MSCs exert their anti-inflammatory effect are unclear there are indications that, in vitro, these cells have an immunomodulatory effect. A number of groups have shown that BM-MSCs inhibit T-lymphocyte proliferation and prevent the differentiation of human monocytes into dendritic cells. This occurs by both secretion of soluble factors and direct cell-cell contact (Zhang et al., (2004); Beyth et al., (2005); Jiang et al., (2005)). There is also evidence that BM-MSCs suppress T-cell activity, as shown by their ability to increase survival in transplant recipients with host-vs-graft disease (Laughlin et al., (2001); Le et al., (2004)). However the precise mechanism by which this happens is unclear. There is some evidence to suggest that direct cell-cell contact is needed (Krampera et al., (2003)) whilst other studies imply a role for soluble factors (Krampera et al., (2006); Di et al., (2002)). It is likely that both systems have a role and further studies are needed to define the exact mechanism.
If, as suggested, BM-MSCs can be mobilised to specific sites in response to an acute injury, terminate the inflammatory response by modulation of cytokine production and contribute to tissue repair, then they make attractive candidates for therapy of acute inflammatory diseases that result in organ damage such as ALI/ARDS. In experimental models of pulmonary hypertension a combination of gene and stem cell therapies has been successful (Zhao et al., (2003); Kugathasan et al., (2005); Zhao et al., (2005)). Human angiopoetin 1 (Ang1) has anti-inflammatory, anti-permeability and endothelial protective properties and down-regulation of this gene has been shown to cause neutrophil infiltration, capillary leakage and pulmonary oedema (Karmpaliotis et al., (2002)). It has also been suggested that it is involved in modulating the response of the pulmonary vessels to injury (McCarter et al., (2007)). This has led to a number of studies looking at the effect of infusing BM-MSCs infected with Ang1 on murine models of endotoxin induced ALI. The overall results suggest that infusion of BM-MSC-Ang1 results in a significant reduction of cell count, neutrophil production and pro-inflammatory cytokines at a level above those achieved with BM-MSCs alone (Xu et al., (2008); Mei et al., (2007)). These results support the use of combined gene-cell therapy for the development of future therapeutic strategies for ALI.
4.1. Summary
Acute lung injury is characterised by pulmonary oedema and impaired gas exchange triggered by an inflammatory response to sepsis
BM-MSC administration in endotoxin induced ALI results in reduced vascular permeability and alveolar oedema mediated by a reduction in the levels of pro-inflammatory cytokines
Immunomodulation via both soluble factors and direct cell-cell contact is thought to result in the down-regulation of the inflammatory response
Experimental models of delivery of combined gene-cell therapy in mice have shown a beneficial effect in reducing inflammation
5. Potential complications of stem cells
Despite the potential benefits of BM-MSCs in lung regeneration and repair there is also evidence to suggest a direct role for these cells in the development of parenchymal fibrosis. In studies using a bleomycin model of lung fibrosis up to 80% of the collagen-producing fibroblasts in the lung were found to be bone marrow-derived (Hashimoto et al., (2004)) whilst another study showed that administration of intravenous BM-MSCs following lung irradiation contributed to fibroblasts and myofibroblasts in areas of damage (Epperly et al., (2003)). Circulating fibrocytes are also recruited by the murine lung in response to a bleomycin challenge resulting in collagen deposition, an effect that is ameliorated by administration of anti-CXCL12 antibodies with resulting reduction in fibrotic response (Phillips et al., (2004)).
There is also a concern that the defining characteristic of stem cells, unlimited self-renewal, could make them a candidate for malignant change (Jordan et al., (2006)), and there is some evidence that these cells develop karyotype abnormalities during in vitro culture (Rubio et al., (2005)). In addition BM-MSC delivery in a murine model has been associated with sarcoma development within the lung parenchyma (Aguilar et al., (2007); Tolar et al., (2007)). The addition of BM-MSCs to human breast carcinoma cells in a mouse subcutaneous xenograft model led to an increased rate of metastasis that was attributed to the de novo secretion of CCL5 from the cells acting in a paracrine fashion causing increased motility and invasion of the malignant cells (Karnoub et al., (2007)). This influence is reversible and dependent on continued CCL5 secretion. It has also been suggested that the immunosuppressive effects of BM-MSCs favour tumour growth although the precise mechanism underlying this is not clear (Djouad et al., (2003)). This propensity to tumorigenesis has been shown in other tumour types (Houghton et al., (2004)) but is not uniform and is likely to be dependent on both tumour model and location (Sangai et al., (2005)).
6. Stem cells as vectors
The ability of bone marrow derived stem cells to migrate to site specific areas in a range of pathological conditions and subsequently provide both structural and functional support suggest that they may be ideal vectors for therapeutic delivery. BM-MSCs possess a number of properties that make them suitable candidates. They are easily obtained from a simple bone marrow aspirate and can be readily expanded in culture to hundreds of millions of cells without losing their multi-lineage potential. They are easily transfectable, allowing for simple ex vivo modification. Finally, they appear to be relatively non-immunogenic (Le Blanc et al., (2003)) due to their expression of major histocompatibility complex 1 (MHC1) but lack of MHC2 and co-stimulatory molecules CD80, CD86 and CD40 (Javazon et al., (2004)). This may allow the delivery of genetically dissimilar MSCs without the need for immunomodulation or subsequent immunosuppressive therapy for the recipient. Because of these properties, MSCs have considerable therapeutic potential in several disease processes, including cardiovascular, respiratory and skeletal disease (see Figure 2).
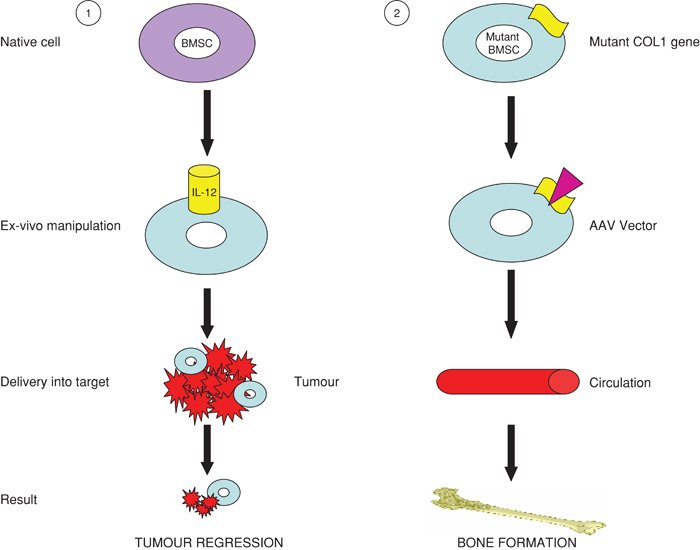
Figure 2. Schematic representation of the use of BMSCs as vectors. (1) Healthy BMSCs are modulated to express anti-proliferative or proapoptotic agents then delivered to tumour cells resulting in tumour cell death. (2) Genetically abnormal BMSCs are transfected with a viral vector resulting in inactivation of the defective gene. These cells are injected back into the subject and normal tissue growth occurs.
6.1. Respiratory disease
6.1.1. Lung cancer
In the case of malignancy, stem cells provide two possible therapeutic options. Either they can be used as inert vehicles for delivery of therapeutics or they can exert a direct influence on tumour progression. There have been a number of studies looking at both of these roles.
Human BM-MSCs expressing interferon beta (IFN-ß) have been used to provide targeted delivery of this antiproliferative and proapoptotic agent to both gliomas (Nakamizo et al., (2005)) and metastatic breast (Studeny et al., (2004)) and melanoma (Studeny et al., (2002)) models with a subsequent increase in survival. Similar cells secreting interleukin-12 (IL-12) have also been injected into mice prior to tumour inoculation and prevented the development of melanoma, lung cancer and hepatocellular carcinoma (Chen et al., (2006)). Similarly murine BM-MSCs transduced to express the immunostimulatory chemokine CX3CL1, were shown to reduce the metastatic load of melanoma and colorectal cancer cell lines and subsequently prolong survival (Xin et al., (2007)).
6.1.2. Non-malignant lung disease
There is also the possibility of using stem cells as genetic vectors in diseases needing protein or DNA replacement. Within respiratory medicine the best example of such a disease is cystic fibrosis. MSCs transduced to express the normal CF transmembrane conductance regulator (CFTR) were mixed with cells from patients with cystic fibrosis in a human airway-epithelial culture. The stem cells differentiated into airway epithelial cells resulting in partial correction of the defective CFTR-dependent chloride channel (Wang et al., (2005)). In vivo, wild type bone marrow cells have been shown to engraft in the lung of CFTR knock-out mice and acquire epithelial phenotypes including CFTR mRNA expression. However, this was a rare occurrence and unlikely to correspond to a clinically significant outcome (Loi et al., (2006)).
What has shown more promise is the use of gene therapy in a mouse model of pulmonary hypertension. A rat monocrotaline-induced pulmonary arterial hypertension (PAH) model was used to look at the effect of endothelial-like progenitor cells (EPCs) on vascular remodelling and pulmonary haemodynamics. EPCs were shown to prevent the development of PAH whilst those transduced with human endothelial NO-synthase (eNOS) resulted in reversal of established disease and significantly improved survival (Zhao et al., (2005); Kanki-Horimoto et al., (2006)). It was postulated in the rat that MSCs act via a paracrine effect (Baber et al., (2007)). The uses of EPCs in the treatment of PAH are currently undergoing phase 1 clinical trials.
6.2. Summary
Stem cells are easily obtained and transfected, readily expanded ex vivo and relatively non-immunogenic making them ideal candidates as vectors
They have been used to deliver anti-proliferative and proapoptotic agents to a variety of malignant lesions causing a reduction in tumour load and greater survival
eNOS transfected BMSCs reverse established PAH and improve survival in a rat model
7. Conclusion
Bone marrow derived stem cells are mobilised in response to tissue injury, migrate to these areas and contribute to tissue repair. Whilst the exact mechanisms remain unclear there is considerable excitement about the potential use of these cells as vectors both for direct genetic therapy and as inert vehicles for delivery of therapeutics. The most promising work on respiratory diseases has been in lung cancer and pulmonary arterial hypertension and early clinical trials are currently underway.
Copyright: © 2008 Elizabeth K. Sage, Michael R. Loebinger, Julia Polak and Sam M. Janes.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
* To whom Correspondence should be addressed. E-mail: s.janes@ucl.ac.uk
* Edited by Sangeeta Bhatia. Last revised August 17, 2008. Published September 30, 2008. This chapter should be cited as: Sage, E.K., Loebinger, M.R., Polak, J. and Janes, S.M., The role of bone marrow-derived stem cells in lung regeneration and repair (September 30, 2008), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.20.1, https://www.stembook.org .
References
- Abe, R. Donnelly, S.C. Peng, T. Bucala, R. Metz, C.N. (2001). Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J. Immunol. 166, 7556–7562.
- Aguilar, S. (2007). Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells 25, 1586–1594.
- Aliotta, J.M. (2006). Bone marrow production of lung cells: The impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp. Hematol. 34, 230–241.
- Anderson, S.A. (2005). Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood 105, 420–425.
- Anjos-Afonso, F. Siapati, E.K. Bonnet, D. (2004). In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 117, 5655–5664.
- Baber, S.R. (2007). Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am. J. Physiol Heart Circ. Physiol 292, H1120–H1128.
- Barth, P.J. Ramaswamy, A. Moll, R. (2002). CD34(+) fi brocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch. 441, 564–568.
- Beyth, S. (2005). Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105, 2214–2219.
- Bhowmick, N.A. Neilson, E.G. Moses, H.L. (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337.
- Bucala, R. Spiegel, L.A. Chesney, J. Hogan, M. Cerami, A. (1994). Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1, 71–81.
- Burger, M. (2003). Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene 22, 8093–8101.
- Cardone, A. Tolino, A. Zarcone, R. Borruto, C.G. Tartaglia, E. (1997). Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 39, 174–177.
- Chamberlain, G. Fox, J. Ashton, B. Middleton, J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25, 2739–2749.
- Chang, J.C. Summer, R. Sun, X. Fitzsimmons, K. Fine, A. (2005). Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am. J. Respir. Cell Mol. Biol. 33, 335–342.
- Chen, X.C. (2006). Prophylaxis against carcinogenesis in three kinds of unestablished tumor models via IL12-gene-engineered MSCs. Carcinogenesis 27, 2434–2441.
- Chute, J.P. (2006). Stem cell homing. Curr. Opin. Hematol. 13, 399–406.
- De Wever, O. Mareel, M. (2003). Role of tissue stroma in cancer cell invasion. J. Pathol. 200, 429–447.
- Desmouliere, A. Guyot, C. Gabbiani, G. (2004). The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int. J. Dev. Biol. 48, 509–517.
- Di, N.M. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99, 3838–3843.
- Direkze, N.C. (2003). Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 21, 514–520.
- Direkze, N.C. (2004). Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 64, 8492–8495.
- Direkze, N.C. Alison, M.R. (2006). Bone marrow and tumour stroma: An intimate relationship. Hematol. Oncol. 24, 189–195.
- Djouad, F. (2003). Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102, 3837–3844.
- Epperly, M.W. Guo, H. Gretton, J.E. Greenberger, J.S. (2003). Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 29, 213–224.
- Ferrari, N. Glod, J. Lee, J. Kobiler, D. Fine, H.A. (2003). Bone marrow-derived, endothelial progenitor-like cells as angiogenesis-selective gene-targeting vectors. Gene Ther. 10, 647–656.
- Gupta, N. (2007). Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 179, 1855–1863.
- Hartmann, T.N. Burger, M. Burger, J.A. (2004). The role of adhesion molecules and chemokine receptor CXCR4 (CD184) in small cell lung cancer. J. Biol. Regul. Homeost. Agents 18, 126–130.
- Hartmann, T.N. Burger, J.A. Glodek, A. Fujii, N. Burger, M. (2005). CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene 24, 4462–4471.
- Hasebe, T. Sasaki, S. Imoto, S. Ochiai, A. (2000). Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastases of invasive ductal carcinoma of the breast. Am. J. Pathol. 156, 1701–1710.
- Hashimoto, N. Jin, H. Liu, T. Chensue, S.W. Phan, S.H. (2004). Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Invest 113, 243–252.
- Honczarenko, M. (2006). Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24, 1030–1041.
- Houghton, J. (2004). Gastric cancer originating from bone marrow-derived cells. Science 306, 1568–1571.
- Ishii, G. (2003). Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem. Biophys. Res. Commun. 309, 232–240.
- Ishii, G. (2005). In vivo characterization of bone marrow-derived fibroblasts recruited into fibrotic lesions. Stem Cells 23, 699–706.
- Ishizawa, K. (2004). Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 556, 249–252.
- Javazon, E.H. Beggs, K.J. Flake, A.W. (2004). Mesenchymal stem cells: paradoxes of passaging. Exp. Hematol. 32, 414–425.
- Jemal, A. (2008). Cancer statistics. CA Cancer J. Clin. 58, 71–96.
- Jiang, Y. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49.
- Jiang, X.X. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105, 4120–4126.
- Jordan, C.T. Guzman, M.L. Noble, M. (2006). Cancer stem cells. N. Engl. J Med. 355, 1253–1261.
- Kanki-Horimoto, S. (2006). Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation 114, I181–I185.
- Karmpaliotis, D. (2002). Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am. J Physiol Lung Cell Mol. Physiol 283, L585–L595.
- Karnoub, A.E. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449, 557–563.
- Khakoo, A.Y. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J. Exp. Med. 203, 1235–1247.
- Kleeberger, W. (2003). Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am. J. Pathol. 162, 1487–1494.
- Kotton, D.N. (2001). Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128, 5181–5188.
- Kotton, D.N. Fabian, A.J. Mulligan, R.C. (2005). Failure of bone marrow to reconstitute lung epithelium. Am. J. Respir. Cell Mol. Biol. 33, 328–334.
- Krampera, M. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101, 3722–3729.
- Krampera, M. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24, 386–398.
- Krause, D.S. (2001). Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105, 369–377.
- Krause, D.S. (2005). Engraftment of bone marrow-derived epithelial cells. Ann. N. Y. Acad. Sci. 1044, 117–124.
- Kugathasan, L. (2005). Role of angiopoietin-1 in experimental and human pulmonary arterial hypertension. Chest 128, 633S–642S.
- Laughlin, M.J. (2001). Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N. Engl. J Med. 344, 1815–1822.
- Le Blanc, K. Tammik, C. Rosendahl, K. Zetterberg, E. Ringden, O. (2003). HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 31, 890–896.
- Le, B.K. (2004). Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441.
- Loi, R. Beckett, T. Goncz, K.K. Suratt, B.T. Weiss, D.J. (2006). Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am. J. Respir. Crit Care Med. 173, 171–179.
- Lyden, D. (2001). Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7, 1194–1201.
- McCarter, S.D. (2007). Cell-based angiopoietin-1 gene therapy for acute lung injury. Am. J Respir. Crit Care Med. 175, 1014–1026.
- Mei, S.H. (2007). Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS. Med. 4, e269.
- Menon, L.G. (2007). Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells 25, 520–528.
- Moore, B.B. (2006). The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am. J. Respir. Cell Mol. Biol. 35, 175–181.
- Nakamizo, A. (2005). Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 65, 3307–3318.
- National Institute of Clinical Excellence. (2005). CG24 Lung cancer: the diagnosis and treatment of lung cancer.. CG24 , 4–5. http://www.nice.org.uk/nicemedia/pdf/CG024niceguideline.pdf, National Institute of Clinical Excellence. 2008. Ref Type: Report
- Ohuchida, K. (2004). Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 64, 3215–3222.
- Orimo, A. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348.
- Ortiz, L.A. (2003). Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. U. S. A. 100, 8407–8411.
- Ozaki, Y. (2007). Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 16, 119–129.
- Peled, A. (1999). Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4.. Science 283, 845–848.
- Perez, A. Rogers, R.M. Dauber, J.H. (2003). The prognosis of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 29, S19–S26.
- Phillips, R.J. (2003). The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am. J. Respir. Crit Care Med. 167, 1676–1686.
- Phillips, R.J. (2004). Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 114, 438–446.
- Ponte, A.L. (2007). The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 25, 1737–1745.
- Ringe, J. (2007). Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2.. J. Cell Biochem. 101, 135–146.
- Rojas, M. (2005). Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 33, 145–152.
- Rubenfeld, G.D. (2005). Incidence and outcomes of acute lung injury. N. Engl. J Med. 353, 1685–1693.
- Rubio, D. (2005). Spontaneous human adult stem cell transformation. Cancer Res. 65, 3035–3039.
- Sakai, N. (2006). Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc. Natl. Acad. Sci. U. S. A 103, 14098–14103.
- Sangai, T. (2005). Effect of differences in cancer cells and tumor growth sites on recruiting bone marrow-derived endothelial cells and myofibroblasts in cancer-induced stroma. Int. J. Cancer 115, 885–892.
- Schmidt, M. Sun, G. Stacey, M.A. Mori, L. Mattoli, S. (2003). Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J. Immunol. 171, 380–389.
- Strieter, R.M. Gomperts, B.N. Keane, M.P. (2007). The role of CXC chemokines in pulmonary fibrosis. J Clin Invest 117, 549–556.
- Studeny, M. (2002). Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 62, 3603–3608.
- Studeny, M. (2004). Mesenchymal stem cells: Potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 96, 1593–1603.
- Su, L. (2005). Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin. Cancer Res. 11, 8273–8280.
- Suratt, B.T. (2003). Human pulmonary chimerism after hematopoietic stem cell transplantation. Am. J. Respir. Crit Care Med. 168, 318–322.
- Theise, N.D. (2002). Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp. Hematol. 30, 1333–1338.
- Tolar, J. (2007). Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25, 371–379.
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.. (2000). The Acute Respiratory Distress Syndrome Network.. N. Engl. J Med. 342, 1301–1308.
- Wagers, A.J. Sherwood, R.I. Christensen, J.L. Weissman, I.L. (2002). Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259.
- Wang, G. (2005). Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A 102, 186–191.
- Weiss, D.J. (2006). Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc. Am. Thorac. Soc. 3, 193–207.
- Xin, H. (2007). Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells 25, 1618–1626.
- Xu, J. (2007). Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am. J. Physiol Lung Cell Mol. Physiol 293, L131–L141.
- Xu, J. (2007). Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am. J. Respir. Cell Mol. Biol. 37, 291–299.
- Xu, J. (2008). Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 214, 472–481.
- Yamada, M. (2004). Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J. Immunol. 172, 1266–1272.
- Zhang, W. (2004). Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 13, 263–271.
- Zhao, Y.D. Campbell, A.I. Robb, M. Ng, D. Stewart, D.J. (2003). Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ. Res. 92, 984–991.
- Zhao, Y.D. (2005). Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: Efficacy of combined cell and eNOS gene therapy in established disease. Circ. Res. 96, 442–450.