The sensory nervous system of the vertebrate head comprises the three paired sense organs, the eye, ear and olfactory epithelium, and the cranial sensory ganglia. It receives contribution from two cell populations: neural crest cells and sensory placodes. The latter are specialised neurogenic epithelia outside of the central nervous system, which arise from unique multipotential cells in the pre-placodal region. This review summarises our current understanding of how sensory placode progenitors are specified from non-committed embryonic ectoderm and how sensory placodes with characteristic identity are induced from those progenitors. In particular, it focuses on how different signalling pathways converge and are used repeatedly to impart distinct fates.
1. Introduction
The vertebrate head ectoderm contains unique neurogenic regions outside the central nervous system (CNS): the cranial sensory placodes. Like the neural plate, which generates the CNS, they are transient columnar epithelia. They form at distinct rostro-caudal positions next to the neural tube and contribute to the special sense organs associated with hearing, balance, olfaction and vision and to the distal parts of the cranial sensory ganglia (see Figure 1). Their derivatives generate a large variety of cell types ranging from simple lens fibre cells to sensory receptors and neurons. While some, like the otic and olfactory placode, undergo complex patterning events to form adult organs, others are simple neurogenic centres that shed neuroblasts into the underlying mesenchyme. Recent evidence highlights that, despite their diversity, all placodes arise from a common territory of multi-potential progenitors that is set aside during early embryogenesis. Precursors for different placodes undergo a series of cell fate decisions to acquire their unique identity and to generate diverse cell types once a placode is formed. This review concentrates on the molecular mechanisms that specify placode precursors in the embryonic ectoderm and lead to their segregation from other ectodermal derivatives, and the events that generate cell diversity among placode progenitors.
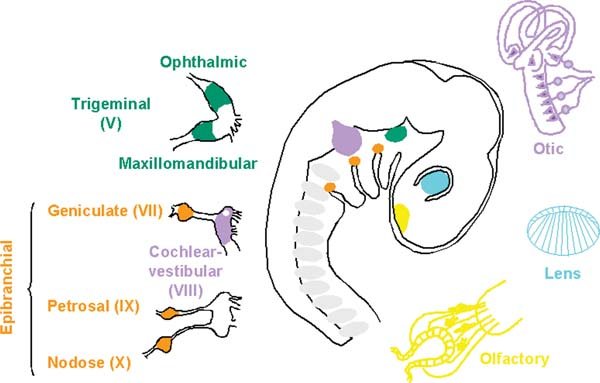
Figure 1. Sensory placodes and their derivatives. The olfactory (yellow), lens (blue), trigeminal (green), otic (purple) and epibranchial (orange) placodes locate to characteristic positions here shown in a side view of a 3-day-old chick embryo. Their derivatives are shown with sense organs on the right and cranial ganglia on the left. Adapted from D’Amico-Martel and Noden, (1983).
2. Sensory placodes and their derivatives
The diversity of adult structures and cell types derived form sensory placodes is immense and is briefly summarised below (see Figure 1; extensive descriptions are found in Webb, (Webb, ., 1993), Baker and Bronner-Fraser, (2001), Schlosser, (2006)). Three placodes contribute to sense organs: the olfactory, lens and otic. The olfactory placode is the most anterior of these and develops next to the forebrain. It generates odorant and pheromone receptor cells that project to the olfactory and accessory olfactory bulb, as well as supporting cells and a diverse set of neurons that express GnRH, somatostatin, neuropeptide Y and calbindin and migrate along the olfactory nerve into the CNS (Murakami and Arai, (1994), Hilal et al., (1996), Mulrenin et al., (1999), Toba et al., (2001), Wray, (2002)). In addition, the olfactory placode contains a population of stem cells that regenerate olfactory neurons throughout life (Farbman, (1994), Schwob, (2002)), which, because of their self renewing capacity, have generated considerable interest as a potential source of adult neural stem cells as have olfactory ensheating cells (Schwob, (2002), Dulac and Zakhary, (2004)). Posterior to the olfactory lies the simplest of the placodes, the lens. This develops next to the future retina and generates the crystalline lens of the eye, composed of only two cell types: lens fibre cells and lens epithelial cells (Lang, (2004), Cvekl and Duncan, (2007)). The lens is the only non-neurogenic sensory placode, but is included here because of its important function in vision and because it is generated from the same territory as the neurogenic placodes. Developing next to the hindbrain, the otic placode generates the entire inner ear including the cochlea, semicircular canals and endolymphatic duct and all associated sensory hair cells, the neurons that innervate them as well as endolymph secreting and supporting cells (Riley and Phillips, (2003), Barald and Kelley, (2004), Ohyama et al., (2007)).
Unlike these three placodes, the trigeminal and epibranchial (geniculate, petrosal and nodose) placodes do not undergo complex morphogenetic events, but are neurogenic patches that produce the distal parts of the trigeminal (Vth), VIIth, IXth and Xth cranial ganglia, respectively (D’Amico-Martel and Noden, (1983), Schlosser and Northcutt, (2000)). While the trigeminal nerve transmits somatosensory information from the head, the epibranchial placode-derived neurons convey gustatory information from the oral cavity, and viscerosensory information from the heart and other visceral organs. The proximal parts of these ganglia are of neural crest cell origin, as are the glial cells that envelop their axons.
3. A unique territory of multipotent sensory progenitors
This brief summary highlights the diversity of cell types generated by sensory placodes as they mature. However, at early developmental stages all placode precursors are located in a common territory, the pre-placodal region (PPR), which is defined by cell fate, gene expression and unique properties common to cells contained in it (reviewed in: Bailey and Streit, (2006), Schlosser, (2006), Streit, (2007)).
While at gastrula stages placode precursors are widely dispersed (Garcia-Martinez et al., (1993), Hatada and Stern, (1994), Streit, unpublished), a continuous placode territory can first be defined at neurula stages (Kozlowski et al., (1997), Streit, (2002), Bhattacharyya et al., (2004), Xu et al., (2008)): all placode precursors locate to a band of ectoderm surrounding the neural plate from forebrain to hindbrain levels (see Figure 2A). Within this domain, precursors for different placodes are initially interspersed, but sort out gradually as development proceeds until unique placodes emerge (see Figure 2B,C). It is not clear whether scattered distribution of precursors with different fates reflects mixing of progenitors with distinct identity that segregate accordingly or whether individual cells are multipotent and differentiate according to signals from their environment. However, since placode cells become committed to their ultimate fate much later and can adopt different identities when exposed to appropriate signals (Waddington, (1937), Jacobson, (1963), Henry and Grainger, (1990), Gallagher et al., (1996), Baker et al., (1999), Groves and Bronner-Fraser, (2000), Bhattacharyya and Bronner-Fraser, (2008); for review: Baker and Bronner-Fraser, (2001)) and since they initially share common characteristics (see below; Bailey et al., (2006), Martin and Groves, (2006)), it seems more likely that the PPR represents a territory of multipotential progenitors. It is noteworthy that even at neurula stages placode precursors overlap medially with both future neural and neural crest cells, however, by the 3–4- somite stage cell fates have segregated with CNS precursors located in the neural plate, neural crest cells in the neural folds and placode progenitors in the non-neural ectoderm (Streit, (2002), Bhattacharyya et al., (2004)).
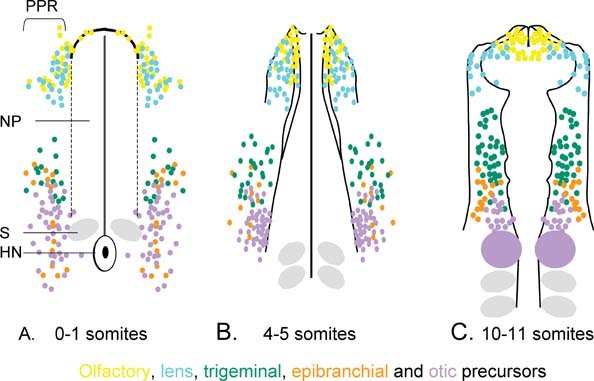
Figure 2. Location of placode precursors at different developmental stages. A. At neurula stages placode precursors are confined to the pre-placodal region (PPR) and surround the anterior neural plate (np). Precursors for different placodes shown by colour-coded dots are intermingled. B. and C. as development proceeds progenitors with different fates segregate. Note: even when the otic placode is morphologically distinct (purple in C.) new cells are still recruited into it. At the 10–12 somite stage precursors for different placodes are largely segregated, although overlap is still observed at territory borders. NP: neural plate; S: somite; HN: Hensen's node. These data are compiled from different fate maps in chick (Streit, (2002), Bhattacharyya, et al., (2004), Xu, et al., (2008)); detailed maps from other species over several stages are not available.
The restriction of cells with different fates to distinct territories is reflected by changes in gene expression (see Figure 3). Before gastrulation the embryonic region co-expresses pre-neural and non-neural genes in partially overlapping areas. However, these domains separate over time and a boundary is generated. Once the definitive neural plate emerges (defined by Sox2), the ectoderm immediately adjacent to it (the “border”) continues to co-express pre-neural (ERNI, Sox3, Geminin; Penzel et al., (1997), Rex et al., (1997), Kroll et al., (1998), Mizuseki et al., (1998), Streit et al., (2000), Kudoh et al., (2004), Papanayotou et al., (2008)) and non-neural markers (Foxi1 genes, Gata2, -3, Dlx5, -3, Tfap2; Papalopulu and Kintner, (1993), Akimenko et al., (1994), Moser et al., (1995), Pera et al., (1999), Sheng and Stern, (1999), Luo et al., (2001), Bakkers et al., (2002), Knight et al., (2003), Liu et al., (2003), Luo et al., (2003), Ohyama and Groves, (2004), Matsuo-Takasaki et al., (2005), Hoffman et al., (2007)), while the future epidermis is devoid of pre-neural genes. Shortly thereafter, Six and Eya gene expression begins in the placode territory (Mishima and Tomarev, (1998), Esteve and Bovolenta, (1999), Kobayashi et al., (2000), Pandur and Moody, (2000), McLarren et al., (2003), Bessarab et al., (2004), Schlosser and Ahrens, (2004), Litsiou et al., (2005), Ishihara et al., (2008)), where Dlx and Foxi1 transcripts are also increased. Finally, neural crest markers (Snail2, Pax7; Nieto et al., (1994), Mayor et al., (1997), Basch et al., (2006)) appear, separating neural and placodal domains. Interestingly, ERNI, which is also expressed in chick embryonic stem cells (Acloque et al., (2001)), has recently been implicated in maintaining cells in an undifferentiated state (Papanayotou et al., (2008)). In vivo, it prevents premature differentiation of neural cells by recruiting co-repressors to the N2 enhancer of the definitive neural marker Sox2. This inhibition is ultimately released by the ERNI-interacting protein Bert. All components of this nuclear complex remain present in PPR until the onset of placode specific gene expression suggesting that PPR cells retain their multipotency.
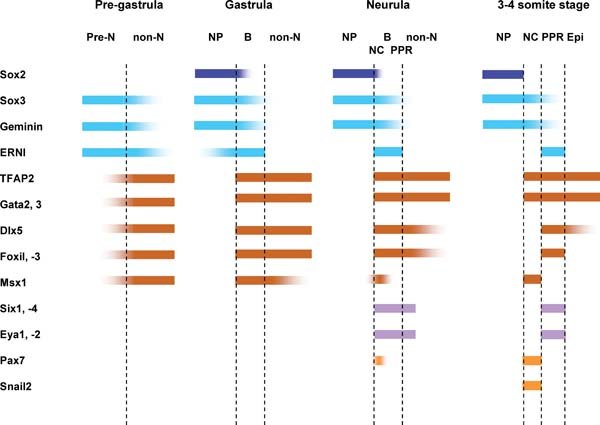
Figure 3. Temporal changes of gene expression reflect segregation of cell fates. At pre-gastrula stages pre-neural (pre-N) and non-neural (non-N) markers partially overlap. The expression domains begin to segregate as the neural plate (NP; Sox2) forms and a border territory (B) is established at its edge, where pre-neural and non neural markers continue to be expressed. Placode precursor specific genes (Six and Eya) are first observed in the border at neurula stages, where they partially overlap with neural crest (NC) markers. By early somite stages, gene expression domains have sharpened and the placode territory (PPR) lies lateral to the neural crest domain. Note: the expression of TFAP2 shown here represents the combined expression of TFAP2a, b and c.
The PPR is defined not only by cell fate and gene expression, but most importantly by unique properties that distinguish it from all other ectodermal derivatives. Only when cells have reached a ‘pre-placodal’ state (i.e. express PPR specific genes) do they have the ability to respond to placode-inducing signals. For example, non-placodal ectoderm is not competent to respond to the otic inducer FGF2 (Martin and Groves, (2006)). However, when grafted into the PPR, the same tissue turns on PPR-specific transcripts and expresses otic markers when exposed to FGF2. These experiments suggest that the PPR indeed is a unique territory and the ‘pre-placodal’ state is a prerequisite for cells to develop into mature placodes.
In addition, cells within the PPR initially share a common developmental program: irrespective of their ultimate fate, at PPR stages they are all specified as lens (Bailey et al., (2006)). When explants from different anterior-posterior PPR levels are cultured in isolation, they initiate lens specific gene expression in a sequence identical to normal lens development and form lens-like structures that express lens-specific crystallins. These findings have two important implications relevant to stem cell research. First, they demonstrate that ectodermal cells contain all information required to execute the lens program already at early developmental stages. Thus, with the emerging knowledge of the signals that induce the PPR (see below), it should be possible to generate lens cells from embryonic or adult stem cells. Second, the data imply that in order to generate placodes other than lens with neurogenic properties, lens differentiation has to be suppressed. The cellular and molecular mechanisms that lead to lens repression will be discussed below.
4. Six and Eya genes and their role in placode development
Of the many transcription factors that are co-expressed in the PPR, only members of the Six and Eya families coincide precisely with the position of placode precursors (Mishima and Tomarev, (1998), Esteve and Bovolenta, (1999), Kobayashi et al., (2000), Pandur and Moody, (2000), McLarren et al., (2003), Bessarab et al., (2004), Schlosser and Ahrens, (2004), Litsiou et al., (2005), Ishihara et al., (2008)). In vertebrates, six Six genes have been identified (Six1–6), while there are only four Eya genes (Eya1–4; for review: Kawakami et al., (2000), Wawersik and Maas, (2000), Hanson, (2001), Rebay et al., (2005)). Six proteins are DNA binding factors that physically interact with Eya, which in turn recruit co-activators to activate downstream target genes (Pignoni et al., (1997), Ohto et al., (1999), Ikeda et al., (2002), Li et al., (2003), Rayapureddi et al., (2003), Hu et al., (2008)). Six proteins can also act as transcriptional repressors by recruiting co-repressors such as Groucho (Zhu et al., (2002), Lopez-Rios et al., (2003)). Cells in the PPR co-express Six1, Six4, Eya1 and Eya2, while a different combination of family members is expressed in each mature placode (Mishima and Tomarev, (1998), Esteve and Bovolenta, (1999), Kobayashi et al., (2000), Pandur and Moody, (2000), McLarren et al., (2003), Bessarab et al., (2004), Schlosser and Ahrens, (2004), Litsiou et al., (2005), Ishihara et al., (2008)).
The importance of Six and Eya genes for sensory organ development was initially demonstrated in Drosophila (for review: Kumar and Moses, (2001), Donner and Maas, (2004), Pappu and Mardon, (2004), Rebay et al., (2005)), where loss of either of the founder members of the Six and Eya families, sine occulis (So) and eyes absent (Eya), causes reduction or complete loss of the eye (Bonini et al., (1993), Cheyette et al., (1994), Mardon et al., (1994), Quiring et al., (1994), Serikaku and O’Tousa, (1994)). In contrast, misexpression of So together with Eya results in the formation of ectopic eyes (Halder et al., (1995), Bonini et al., (1997), Chen et al., (1997), Pignoni et al., (1997), Shen and Mardon, (1997), Weasner et al., (2007)). Since then, loss of Six and Eya function in mouse, zebrafish and humans has demonstrated their involvement in vertebrate sensory organ formation, specifically for cell proliferation and neurogenesis. In particular, inactivation of Six1 or Eya1 results in defects in the ear, cranial ganglia and the olfactory epithelium, while in humans their mutation leads to Branchial-Oto-Renal (BOR) syndrome associated with deafness, renal and branchial malformations (Abdelhak et al., (1997), Johnson et al., (1999), Xu et al., (1999), Azuma et al., (2000), Laclef et al., (2003), Li et al., (2003), Zheng et al., (2003), Ozaki et al., (2004), Ruf et al., (2004), Zou et al., (2004), Friedman et al., (2005), Kozlowski et al., (2005), Whitfield, (2005), Zou et al., (2006)). Thus, normal development of almost all placodes is affected in the absence of Six1 and Eya1 function, reflecting their early widespread expression and a possible function in the PPR. However, none of these studies has addressed an early role of these genes and since members of the same family have similar functions they may compensate for each other. In Xenopus, Six1 promotes the expression of preplacodal markers, while repressing neural crest and neural plate formation (Brugmann et al., (2004)). These findings suggest that the Six/Eya cassette may play a role in specifying placode precursors at early developmental stages. However, unlike in the fly, misexpression of Six1 and Eya2 in vertebrates does not lead to ectopic placode formation outside of the PPR (Christophorou and Streit, unpublished), indicating that additional factors are required and/or that placode formation underlies more complex regulatory mechanisms.
In summary, while there is good evidence that Six and Eya proteins play an important role in different aspects of placode development and cell fate specification within the placodes, further experiments are required to determine their precise role in specifying placode progenitors. In particular, it will be important to identify their immediate transcriptional targets.
5. Induction of the placode territory through combinatorial signalling
As discussed above, sequential gene expression and refinement reflects the subdivision of the ectoderm into regions of progenitors with restricted developmental potential. Multiple signalling pathways control these events and converge to impart distinct properties to sensory placode precursors and to induce Six and Eya gene expression in ectodermal cells.
5.1. FGF pathway
Fgfs have been implicated as the earliest factors that impart pre-neural character before gastrulation by rapidly (within 1–3 hrs) initiating the expression of ERNI, Sox3 and Geminin (Streit et al., (2000), Wilson et al., (2000), Sheng et al., (2003), Linker and Stern, (2004), Delaune et al., (2005), Papanayotou et al., (2008)). In addition, Fgfs can induce the expression of Dlx5 and Msx1 after 12–15 hrs (Streit and Stern, (1999), Litsiou et al., (2005)). All five genes are later co-expressed at the border of the neural plate, which generates placode and neural crest precursors. While FGF signalling is indeed required for border gene expression and for the formation of border derivatives, it is not sufficient to generate them in non-border territory (Mayor et al., (1997), LaBonne and Bronner-Fraser, (1998), Monsoro-Burq et al., (2003), Ahrens and Schlosser, (2005), Litsiou et al., (2005)). In addition, FGF8 induces the expression of the PPR marker Eya2, suggesting that it may play a dual role in specifying placode precursors (Litsiou et al., (2005)). Accordingly, the mesoderm underlying the border and the neural plate adjacent to it express various FGFs (Niswander and Martin, (1992), Shamim and Mason, (1999), Streit and Stern, (1999), Ahrens and Schlosser, (2005)). These observations suggest a role for Fgfs in promoting pre-neural and later border character in ectodermal cells as a prerequisite to generate definitive neural, neural crest and placode cells.
5.2. Bmp pathway
Many studies have implicated Bmp signalling in early ectodermal patterning (Wilson et al., (1997), Marchant et al., (1998), Barth et al., (1999), Tribulo et al., (2003); for review Sasai and De Robertis, (1997), Aybar and Mayor, (2002), Stern, (2005)). One model suggests that a gradient of Bmp activity within the ectoderm allocates different cell fates, with high levels of signalling promoting epidermis, moderate levels inducing placodes, intermediate levels specifying neural crest and complete absence of Bmp activity being required for neural plate formation (for review Sasai and De Robertis, (1997), Aybar and Mayor, (2002), Vonica and Brivanlou, (2006)). Accordingly, Xenopus animal caps treated with Bmp antagonists form epidermis, pre-placodal tissue, neural crest and neural plate, respectively, with increasing concentrations (Wilson et al., (1997), Tribulo et al., (2003), Brugmann et al., (2004), Glavic et al., (2004)). When Bmp signalling is reduced in zebrafish mutants, creating a shallower gradient of activity, the neural crest territory is expanded to a relatively larger extent than the neural plate, while the position of the PPR shifts but its width does not change (Neave et al., (1997), Nguyen et al., (1998), Barth et al., (1999)). Together with a recent study demonstrating that Bmp inhibition at different times of development has distinct outcomes (Wawersik et al., (2005)), these observations are at odds with a simple gradient model.
If not as a gradient, how does Bmp signalling influence ectodermal patterning? Several non-neural markers like FoxiIa, Msx1, Dlx3 and –5, which are expressed early and widespread, are dependent on Bmp activity. In zebrafish Bmp7 and –2a mutants, FoxiI expression is reduced, while in Xenopus expression of the Bmp antagonist Chordin leads to its downregulation (Matsuo-Takasaki et al., (2005), Hans et al., (2007)). In contrast, Bmp4 misexpression expands FoxiI at the expense of neural tissue. Likewise, reduction or absence of Bmp signalling in chick, frog and fish leads to loss of Dlx genes (Nguyen et al., (1998), Feledy et al., (1999), Pera et al., (1999), Luo et al., (2001)), and Msx1, a direct target of Bmp signalling, mediates its ability to promote epidermis (Suzuki et al., (1997)). These data implicate Bmp activity in the regulation of border genes and the positioning of the border.
Indeed, the border itself appears to be the only region sensitive to modulation of Bmp signalling (Streit and Stern, (1999)). Local inhibition of Bmp signalling close to the border expands the neural plate and the PPR, but is not sufficient to induce either in future epidermis (Streit et al., (1998), Glavic et al., (2004), Linker and Stern, (2004), Ahrens and Schlosser, (2005), Delaune et al., (2005), Litsiou et al., (2005)). In fact, PPR markers are expressed in cells expressing exogenous Smad6, a cell-autonomous Bmp inhibitor which, unlike soluble Bmp antagonists, completely abolishes the pathway, suggesting that Bmp activity is not required for the formation of placode progenitors, but indeed needs to be suppressed. This is in direct conflict with a recent study claiming that Bmp signalling induces placode progenitors (Sjodal et al., (2007)). However, since the test tissue used is equivalent to the border region at gastrula stages, it is likely that Bmp signalling promotes border identity which subsequently generates sensory precursors.
In summary, these findings implicate Bmp activity, like Fgf signalling, in the regulation of border-specific genes before placode and neural crest cells are specified. It is noteworthy that at a slightly later point in development Bmp signalling in the neural folds is required for neural crest cell formation. This observation highlights the importance of timing and the fact that interpretation of the same signalling pathway is highly context dependent and is determined by the developmental history of individual cells (for discussion see also: Sheng et al., (2003), Stern, (2005)).
Interestingly, gain- and loss-of-function studies for the border specific transcription factors Foxi1, Dlx3 and –5 and Msx1, which are regulated by Bmp, show the same effect as modulation of Bmp activity: the border of the neural plate is shifted. It is therefore likely that Bmp signalling acts via these mediators to alter pre-placodal gene expression indirectly. Indeed, FoxiI and Dlx genes are required for PPR specification and/or placode development, while Dlx genes have been shown to be sufficient to induce some PPR markers (Luo et al., (2001), Solomon and Fritz, (2002), Lee et al., (2003), McLarren et al., (2003), Nissen et al., (2003), Solomon et al., (2003), Woda et al., (2003), Kaji and Artinger, (2004)).
5.3. Wnt pathway
As discussed above, both Fgfs and Bmps modulate gene expression in the PPR: inhibition of Bmp signalling expands preplacodal markers, while FGF8 promotes the expression of Eya2 (Brugmann et al., (2004), Ahrens and Schlosser, (2005), Litsiou et al., (2005)). However, even when Fgfs are combined with Bmp antagonists, they are not sufficient to induce PPR character in ectoderm away form the endogenous placode territory (Ahrens and Schlosser, (2005), Litsiou et al., (2005)) suggesting that additional signals are required. Inhibition of canonical Wnt signalling enlarges the neural plate (Heeg-Truesdell and LaBonne, (2006)), while Wnt signalling is necessary for neural crest cell development (for review: Aybar and Mayor, (2002), Knecht and Bronner-Fraser, (2002)). When Wnt signalling is modulated pre-placodal cells behave like CNS progenitors: in the presence of Wnt antagonists the PPR expands, while activation of the canonical Wnt pathway prevents PPR specific gene expression (Brugmann et al., (2004), Litsiou et al., (2005)). Thus, in the border region the level of Wnt signalling determines whether cells adopt placode or neural crest fate.
In summary, temporal and spatial integration of different signals results in the specification of placode precursors characterised by the co-expression of Six1, Six4, Eya1 and Eya2. FGFs initially promote a pre-neural state that is common to neural, neural crest and placode precursors and in which cells are multipotent. Bmp signalling promotes non-neural character and limits the size of the neural plate. Together with Fgfs, Bmp activity maintains and up-regulates border gene expression and local modulation of Wnt signalling determines whether cells within the border are specified as neural crest or placode precursors. Overall, the signals required for placode progenitors specification are akin to those that promote neural identity raising the possibility that both populations share some of the early inducing steps.
6. Specification of individual placodes from common field
6.1. Sequential subdivision of the placode territory
So far the discussion has focused on the properties and induction of placode progenitors. The remaining part of the review will summarise how, once the PPR is established, placodes with distinct identities emerge. Shortly after PPR induction, differential expression of several transcription factors is observed along its anterior-posterior axis, reflecting its regional subdivision (see Figure 4). As development proceeds more factors become expressed in smaller sub-domains until eventually each placode is defined by a unique transcription factor code. These changes in gene expression have recently been reviewed elsewhere (Schlosser, (2006)) and a detailed description is beyond the scope of this review. Suffice it to say, however, that many of the factors involved have already been implicated in imparting regional identity onto cells of the neural plate. Future experiments will need to address whether they have similar functions in the PPR and are responsible for sequential subdivision of the placode territory.
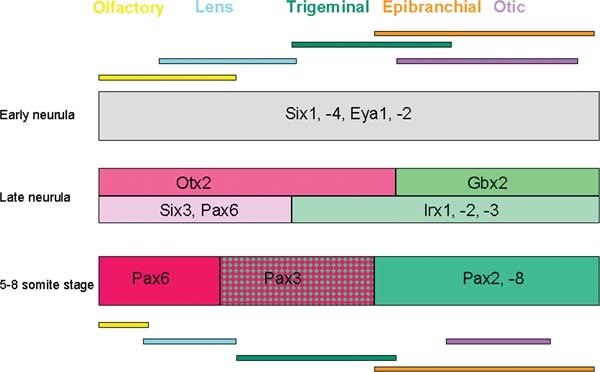
Figure 4. Sequential subdivision of the placode territory. Once established the PPR becomes sequentially subdivided into smaller domains reflected by differential gene expression. This diagram shows only a few examples of the earliest events. Colour-coded bars at the top and bottom indicate the position of placode precursors at neurula and early somite stages, respectively. Anterior is to the left and posterior to the right of the diagram.
What are the signalling pathways that control regionalisation in the PPR? Some observations suggest that the signals may be similar to those that pattern the neural plate. For example, in zebrafish masterblind mutants, which have increased Wnt activity due to loss of axin, anterior forebrain structures are lost (Heisenberg et al., (1996), Heisenberg et al., (2001)). In addition, the most anterior placodes, lens and olfactory, are absent, while the trigeminal territory appears to be expanded. Likewise, the posteriorising factors FGF and RA have been implicated in formation of the most caudal placode, the otic primordium (Ladher et al., (2000), Vendrell et al., (2000), Phillips et al., (2001), Leger and Brand, (2002), Maroon et al., (2002), Liu et al., (2003), Wright and Mansour, (2003), Hans et al., (2007), Hans and Westerfield, (2007), Zelarayan et al., (2007)). Whether or not all of these signalling pathways are active in the placode territory itself or whether they act indirectly through patterning of the neural plate remains to be elucidated.
6.2. Lens suppression by Fgfs and neural crest cells
Initially all placode precursors share common properties: irrespective of their final fate, they are specified as lens (see Figure 5A). This finding implies that, to generate neurogenic placodes, lens specification needs to be suppressed. Fgf signalling seems to play an important role in initiating this process. Exposure of pre-placodal ectoderm to FGF8 leads to rapid loss of the early lens marker Pax6 (Bailey et al., (2006)). In addition, activation of the FGF pathway promotes olfactory identity, while depending on species FGF2, −3, −8, −10 and −19 have been implicated in otic induction and FGF3 and −8 in the induction of epibranchial placodes (Ladher et al., (2000), Vendrell et al., (2000), Phillips et al., (2001), Leger and Brand, (2002), Maroon et al., (2002), Liu et al., (2003), Wright and Mansour, (2003), Nechiporuk et al., (2005), Bailey et al., (2006), Hans et al., (2007), Hans and Westerfield, (2007), Nechiporuk et al., (2007), Nikaido et al., (2007), Sun et al., (2007), Zelarayan et al., (2007)). It therefore seems that Fgf signalling plays a crucial role in suppressing lens and promoting the formation of other, non-lens placodes (see Figure 5B).
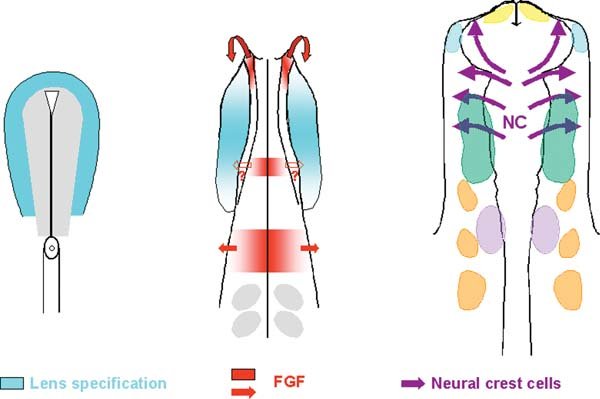
Figure 5. A model for lens repression. At neurula stages the entire placode territory is specified as lens (blue). Initially Fgfs from the anterior neural ridge, the future hindbrain and the mesoderm underlying the otic placode (not indicated in the diagram) repress lens specification. Fgf8 is also expressed in the future mid-hindbrain boundary, however a role in lens suppression and trigeminal placode specification has not yet been shown (empty arrow). Neural crest cells come to underlie most of the facial ectoderm and are potent lens suppressors (purple arrows).
These observations, however, raise the question of how activation of the same signalling pathway leads to fundamentally different outcomes and the specification of different sensory structures. One important aspect is timing. For example, while in zebrafish inhibition of Fgf signalling during gastrulation prevents the formation of the otic (Phillips et al., (2001), Maroon et al., (2002)), inhibition at later stages using the same reagents leaves the otic primordium intact but suppresses the development of epibranchial placodes (Nechiporuk et al., (2007)). One possibility is that otic and epibranchial precursors initially form a common territory, which is then further subdivided by additional signals (Nechiporuk et al., (2007), Sun et al., (2007)). In fact, recent studies in the chick have implicated Fgfs in the induction of this common primordium (Freter et al., (2008)). While continued otic specification subsequently requires the absence of FGF signalling and reinforcement by a combination of Wnt and Notch, epibranchial precursors continue to depend on FGF alone (Ohyama et al., (2006), Jayasena et al., (2008); Freter et al., (2008)). Thus, length or level of Fgf exposure determines the final outcome. Finally, as highlighted above, regionalisation of the PPR begins already shortly after its induction. Thus, at the time when Fgfs act to specify different placodes, cells within the PPR have already acquired some regional identity reflected by differential expression of transcription factors that may act in concert with Fgf targets to impart cell identity.
Recent data also reveal that while Fgf is crucial for initiating lens suppression, it is not sufficient to prevent lens formation in all non-lens ectoderm, suggesting that additional signals are required (Bailey et al., (2006)). These signals emanate from neural crest cells, which come to underlie most of the facial ectoderm except for the lens. Co-culture experiments reveal that neural crest cells prevent lens formation from presumptive lens ectoderm. In contrast, ablation of neural crest cells leads to ectopic lens formation, suggesting that indeed neural crest cells are potent lens inhibitors. The signals responsible for this activity have not yet been identified. While neural crest cells do not appear to instruct cranial ectoderm to differentiate into specific placodes, they play a role in patterning the olfactory placode once it is established (LaMantia et al., (2000), Bhasin et al., (2003)).
Lens specification at pre-placodal stages has so far only been demonstrated in the chick, while the inhibitory influence of neural crest cells has been observed in chick as well as newts (von Woellwarth, (1961), Bailey et al., (2006)). In contrast, lens specification in mouse and Xenopus appears to occur only after contact with the optic vesicle (Henry and Grainger, (1990), Grainger et al., (1997), Furuta and Hogan, (1998)). One possible explanation for these differences is that in chick neural crest cells are never in contact with the future lens ectoderm, while in mouse and Xenopus they are. In chick the optic vesicle makes close contact with the future lens shortly after the onset of neural crest migration and thus provides a mechanical barrier (Bellairs and Osmond, (1998)). On the other hand in mouse and Xenopus, direct contact between the optic vesicle and lens ectoderm is established rather late and mesenchymal cells that may be of crest origin underlie the lens territory (Kaufman, (1979), Nieuwkoop and Faber, (1994)). This in turn may prevent its early specification. Future experiments will need to address this issue in different species.
6.3. Signalling pathways in placode induction
As illustrated above Fgf signalling plays an important role in the induction of olfactory, epibranchial and otic placodes from placode progenitors. However, conferring definitive placode identity generally entails different sequential or simultaneous signals.
Otic induction by Fgfs is well documented through loss- and gain-of-function approaches in mouse, zebrafish and chick (see above). These studies clearly demonstrate a crucial role for this pathway. However, once early otic genes like Pax2 are expressed, Pax2+ cells only continue along the otic pathway, when Fgf signalling is reduced (Freter et al., (2008)) with. In addition, they require reinforcement through Wnt and Notch signalling (Ohyama et al., (2006), Jayasena et al., (2008)). When canonical Wnt signalling is inhibited in Pax2+ cells, they revert to epidermis, while activation of Wnt leads to a larger placode. In this context, Notch signalling cooperates with Wnt to promote otic character.
Fgfs also mediate epibranchial placode induction and one model suggests that epibranchial precursors initially form a common territory with otic cells, but later diversify (Nechiporuk et al., (2005), Nechiporuk et al., (2007), Nikaido et al., (2007), Sun et al., (2007)). Recent evidence suggests that unlike otic progenitors, epibranchial precursors require continued Fgf signalling, but are inhibited in the presence of Wnt activity. Thus the level or time of exposure to Wnts determines whether cells follow otic or epibranchial fates. Epibranchial neurogenesis is dependent on Bmp signals from the underlying endoderm (Begbie et al., (1999), Holzschuh et al., (2005)).
So far, Fgf signalling has not been implicated in trigeminal placode induction. In contrast, PDGF and canonical Wnt signalling are required for initiation of the early trigeminal marker Pax3 and subsequently for trigeminal neurogenesis (Lassiter et al., (2007), McCabe and Bronner-Fraser, (2008)). While activation of the Wnt pathway does not expand the placode territory, ectopic expression of PDGF increases the number of Pax3+ cells. These data suggest that while Wnt signalling is active in the trigeminal ectoderm and required for its formation, it is not sufficient to confer trigeminal character onto placode precursors. Importantly, PDGF is only sufficient to augment the number of trigeminal cells, but not to induce them ectopically (McCabe and Bronner-Fraser, (2008)) indicating that additional signals are required for trigeminal specification.
Although Fgfs initially suppress lens character, at placode stages Fgf signalling is important for continued lens development (Faber et al., (2001)). In addition, Bmp activity is required for the activation of placode specific gene expression like Sox2 and placodal Pax6 (Furuta and Hogan, (1998), Wawersik et al., (1999)). A recent study in chick has implicated Bmp signalling in lens specification from gastrula stage ectoderm (Sjodal et al., (2007)). However, since the test tissue contains a mixture of different progenitors including future retina, neural plate and border territory, it remains unclear whether this activity is truly an instructive induction or whether it is indirect, acting through modulation of other precursors.
Finally, Fgf signalling is sufficient and required to generate olfactory placodes from pre-placodal ectoderm (Bailey et al., (2006)). The same study that had implicated Bmp signalling in early lens specification (Sjodal et al., (2007)) also suggested that Bmps are involved in olfactory placode specification, with low levels or shorter exposure to Bmps favouring olfactory versus lens differentiation. However, as outlined above, it is not certain that in this scenario Bmps act directly on placode precursors or on different progenitors which in turn may induce olfactory cells.
7. Conclusions
This review highlights that specification of sensory placodes is a complex process that begins before gastrulation and involves a series of cell fate decision before multi-potential placode progenitors are specified in the ectoderm next to the neural plate (see Figure 6). During this process, future placode cells first acquire a pre-neural identity, which they share with neural and neural crest cell precursors. Subsequently, they acquire a border state characteristic for cells in the immediate vicinity of the neural plate, from which placode precursors eventually emerge. Once the placode territory has been established, localised, sequential signalling events lead to the repression of lens specification and the induction of neurogenic placodes with distinct identities. These processes are controlled by a number of pathways including Fgf, Bmp and Wnt signalling, which act repeatedly at different times of sensory placode specification. The process of placode specification is accompanied by the increasingly complex array of transcription factors that ultimately appear to encode placode identity. In the future, our understanding of how cell fate decision are controlled to generate sensory progenitors will be useful to direct stem cells towards specific fates such as auditory hair cells or different sublcasses of sensory neurons.
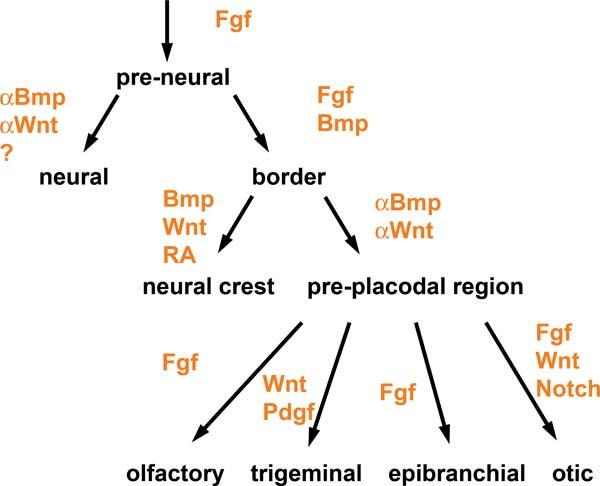
Figure 6. Signalling pathways in placode formation. The diagram summarises the signalling pathways that have been implicated in the specification of the pre-placodal region and in the induction of placodes from the pre-placodal region. Note: the pre-placodal region is initially specified as lens.
Copyright: © 2008 Andrea Streit.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
To whom correspondence should be addressed. E-mail: andrea.streit@kcl.ac.uk
* Edited by Fiona Watt and Fred Gage. Last revised November 20, 2008. Published December 15, 2008. This chapter should be cited as: Streit, A., The cranial sensory nervous system: specification of sensory progenitors and placodes (December 15, 2008), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.31.1, https://www.stembook.org .
References
- Abdelhak, S. Kalatzis, V. Heilig, R. Compain, S. Samson, D. Vincent, C. Weil, D. Cruaud, C. Sahly, I. Leibovici, M. et al. (1997). A human homologue of the Drosophila eyes absent gene underlies branchio- oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet 15, 157–164. Abstract DOI
- Acloque, H. Risson, V. Birot, A.-M. Kunita, R. Pain, B. Samarut, J. (2001). Identification of a new gene family specifically expressed in chicken embryonic stem cells and early embryo. Mech Dev 103, 79–91. Abstract DOI
- Ahrens, K. Schlosser, G. (2005). Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol 288, 40–59. Abstract DOI
- Akimenko, M.A. Ekker, M. Wegner, J. Lin, W. Westerfield, M. (1994). Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J Neurosci 14, 3475–3486. Abstract Abstract
- Aybar, M.J. Mayor, R. (2002). Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev 12, 452–458. Abstract DOI
- Azuma, N. Hirakiyama, A. Inoue, T. Asaka, A. Yamada, M. (2000). Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet 9, 363–366. Abstract DOI
- Bailey, A.P. Bhattacharyya, S. Bronner-Fraser, M. Streit, A. (2006). Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell 11, 505–517. Abstract DOI
- Bailey, A.P. Streit, A. (2006). Sensory organs: making and breaking the pre-placodal region. In Curr Top Dev Biol , 167–204.
- Baker, C.V. Stark, M.R. Marcelle, C. Bronner-Fraser, M. (1999). Competence, specification and induction of Pax-3 in the trigeminal placode. Development 126, 147–156. Abstract Abstract
- Baker, C.V. Bronner-Fraser, M. (2001). Vertebrate cranial placodes I. Embryonic induction. Dev Biol 232, 1–61. Abstract DOI
- Bakkers, J. Hild, M. Kramer, C. Furutani-Seiki, M. Hammerschmidt, M. (2002). Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell 2, 617–627. Abstract DOI
- Barald, K.F. Kelley, M.W. (2004). From placode to polarization: new tunes in inner ear development. Development 131, 4119–4130. Abstract DOI
- Barth, K.A. Kishimoto, Y. Rohr, K.B. Seydler, C. Schulte-Merker, S. Wilson, S.W. (1999). Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development 126, 4977–4987. Abstract Abstract
- Basch, M.L. Bronner-Fraser, M. Garcia-Castro, M.I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441, 218–222. Abstract DOI
- Begbie, J. Brunet, J.F. Rubenstein, J.L. Graham, A. (1999). Induction of the epibranchial placodes. Development 126, 895–902. Abstract Abstract
- Bellairs, R. Osmond, M.K. (1998). The atlas of chick development. Academic Press;
- Bessarab, D.A. Chong, S.W. Korzh, V. (2004). Expression of zebrafish six1 during sensory organ development and myogenesis. Dev Dyn 230, 781–786. Abstract DOI
- Bhasin, N. Maynard, T.M. Gallagher, P.A. LaMantia, A.S. (2003). Mesenchymal/epithelial regulation of retinoic acid signaling in the olfactory placode. Dev Biol 261, 82–98. Abstract DOI
- Bhattacharyya, S. Bailey, A.P. Bronner-Fraser, M. Streit, A. (2004). Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol 271, 403–414. Abstract DOI
- Bhattacharyya, S. Bronner-Fraser, M. (2008). Competence, specification and commitment to an olfactory placode fate. Development 135, 4165–4177. Abstract DOI
- Bonini, N.M. Leiserson, W.M. Benzer, S. (1993). The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72, 379–395. Abstract DOI
- Bonini, N.M. Bui, Q.T. Gray-Board, G.L. Warrick, J.M. (1997). The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development 124, 4819–4826. Abstract Abstract
- Brugmann, S.A. Pandur, P.D. Kenyon, K.L. Pignoni, F. Moody, S.A. (2004). Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development 131, 5871–5881. Abstract DOI
- Chen, R. Amoui, M. Zhang, Z. Mardon, G. (1997). Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye development in Drosophila [see comments]. Cell 91, 893–903. Abstract DOI
- Cheyette, B.N. Green, P.J. Martin, K. Garren, H. Hartenstein, V. Zipursky, S.L. (1994). The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12, 977–996. Abstract DOI
- Cvekl, A. Duncan, M.K. (2007). Genetic and epigenetic mechanisms of gene regulation during lens development. Prog Retin Eye Res 26, 555–597. Abstract DOI
- Delaune, E. Lemaire, P. Kodjabachian, L. (2005). Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development 132, 299–310. Abstract DOI
- Donner, A.L. Maas, R.L. (2004). Conservation and non-conservation of genetic pathways in eye specification. Int J Dev Biol 48, 743–753. Abstract DOI
- Dulac, C. Zakhary, L. (2004). Stem Cells of the Olfactory Epithelium Handbook of Stem Cells Lanza, R. Gearhart, J. Hogan, B. Melton, D. Pedersen, R. Thomson, J. West, M. Burlington: Academic Press; , 233–244.
- D’Amico-Martel, A. Noden, D.M. (1983). Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat 166, 445–468. Abstract DOI
- Esteve, P. Bovolenta, P. (1999). cSix4, a member of the six gene family of transcription factors, is expressed during placode and somite development. Mech Dev 85, 161–165. Abstract DOI
- Faber, S.C. Dimanlig, P. Makarenkova, H.P. Shirke, S. Ko, K. Lang, R.A. (2001). Fgf receptor signaling plays a role in lens induction. Development 128, 4425–4438. Abstract Abstract
- Farbman, A.I. (1994). Developmental biology of olfactory sensory neurons. Semin Cell Biol 5, 3–10. Abstract DOI
- Feledy, J.A. Beanan, M.J. Sandoval, J.J. Goodrich, J.S. Lim, J.H. Matsuo-Takasaki, M. Sato, S.M. Sargent, T.D. (1999). Inhibitory patterning of the anterior neural plate in Xenopus by homeodomain factors Dlx3 and Msx1. Dev Biol 212, 455–464. Abstract DOI
- Freter, S. Muta, Y. Mak, S. Rinkwitz, S. Ladher, R.J. (2008). Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development 135, 3415–3424. Abstract DOI
- Friedman, R.A. Makmura, L. Biesiada, E. Wang, X. Keithley, E.M. (2005). Eya1 acts upstream of Tbx1, Neurogenin 1, NeuroD and the neurotrophins BDNF and NT-3 during inner ear development. Mech Dev 122, 625–634. Abstract DOI
- Furuta, Y. Hogan, B.L. (1998). BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12, 3764–3775. Abstract DOI
- Gallagher, B.C. Henry, J.J. Grainger, R.M. (1996). Inductive processes leading to inner ear formation during Xenopus development. Dev Biol 175, 95–107. Abstract DOI
- Garcia-Martinez, V. Alvarez, I.S. Schoenwolf, G.C. (1993). Locations of the ectodermal and nonectodermal subdivisions of the epiblast at stages 3 and 4 of avian gastrulation and neurulation. J Exp Zool 267, 431–446. Abstract DOI
- Glavic, A. Maris Honore, S. Gloria Feijoo, C. Bastidas, F. Allende, M.L. Mayor, R. (2004). Role of BMP signaling and the homeoprotein Iroquois in the specification of the cranial placodal field. Dev Biol 272, 89–103. Abstract DOI
- Grainger, R.M. Mannion, J.E. Cook, T.L. Zygar, C.A. (1997). Defining intermediate stages in cell determination: acquisition of a lens-forming bias in head ectoderm during lens determination. Dev Genet 20, 246–257. Abstract Abstract
- Groves, A.K. Bronner-Fraser, M. (2000). Competence, specification and commitment in otic placode induction. Development 127, 3489–3499. Abstract Abstract
- Halder, G. Callaerts, P. Gehring, W. J. (1995). Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science 267, 1788–1792. Abstract DOI
- Hans, S. Christison, J. Liu, D. Westerfield, M. (2007). Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol 7, 5. Abstract DOI
- Hans, S. Westerfield, M. (2007). Changes in retinoic acid signaling alter otic patterning 10.1242/dev.000448. Development 134, 2449–2458. Abstract DOI
- Hanson, I.M. (2001). Mammalian homologues of the Drosophila eye specification genes. Semin Cell Dev Biol 12, 475–484. Abstract DOI
- Hatada, Y. Stern, C. D. (1994). A fate map of the epiblast of the early chick embryo. Development 120, 2879–2889. Abstract Abstract
- Heeg-Truesdell, E. LaBonne, C. (2006). Neural induction in Xenopus requires inhibition of Wnt-[beta]-catenin signaling. Dev Biol 298, 71–86. Abstract DOI
- Heisenberg, C.P. Brand, M. Jiang, Y.J. Warga, R.M. Beuchle, D. van Eeden, F.J. Furutani-Seiki, M. Granato, M. Haffter, P. Hammerschmidt, M. et al. (1996). Genes involved in forebrain development in the zebrafish, Danio rerio. Development 123, 191–203. Abstract Abstract
- Heisenberg, C.P. Houart, C. Take-Uchi, M. Rauch, G.J. Young, N. Coutinho, P. Masai, I. Caneparo, L. Concha, M.L. Geisler, R. et al. (2001). A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev 15, 1427–1434. Abstract DOI
- Henry, J.J. Grainger, R.M. (1990). Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev Biol 141, 149–163. Abstract DOI
- Hilal, E.M. Chen, J.H. Silverman, A.J. (1996). Joint migration of gonadotropin-releasing hormone (GnRH) and neuropeptide Y (NPY) neurons from olfactory placode to central nervous system. J Neurobiol 31, 487–502. Abstract Abstract
- Hoffman, T.L. Anna, L.J. Campeau, SA. Knight, RD. Schilling, TF. (2007). Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J Exp Zool Part B: Mol Dev Evol 308B, 679–691. Article DOI
- Holzschuh, J. Wada, N. Wada, C. Schaffer, A. Javidan, Y. Tallafuss, A. Bally-Cuif, L. Schilling, T.F. (2005). Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development 132, 3731–3742. Abstract DOI
- Hu, S. Mamedova, A. Hegde, R.S. (2008). DNA-Binding and Regulation Mechanisms of the SIX Family of Retinal Determination Proteins. Biochemistry 47, 3586–3594. Abstract DOI
- Ikeda, K. Watanabe, Y. Ohto, H. Kawakami, K. (2002). Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol Cell Biol 22, 6759–6766. Abstract DOI
- Ishihara, T. Ikeda, K. Sato, S. Yajima, H. Kawakami, K. (2008). Differential expression of Eya1 and Eya2 during chick early embryonic development. Gene Expr Patterns 8, 357–367. Abstract DOI
- Jacobson, A. (1963). The determination and positioning of the nose, lens and ear. J Exp Zool 154, 273–303. Abstract DOI
- Jayasena, C.S. Ohyama, T. Segil, N. Groves, A.K. (2008). Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode 10.1242/dev.017905. Development 135, 2251–2261. Abstract DOI
- Johnson, K.R. Cook, S.A. Erway, L.C. Matthews, A.N. Sanford, L.P. Paradies, N.E. Friedman, R.A. (1999). Inner ear and kidney anomalies caused by IAP insertion in an intron of the Eya1 gene in a mouse model of BOR syndrome. Hum Mol Genet 8, 645–653. Abstract DOI
- Kaji, T. Artinger, K.B. (2004). dlx3b and dlx4b function in the development of Rohon-Beard sensory neurons and trigeminal placode in the zebrafish neurula. Dev Biol 276, 523–540. Abstract DOI
- Kaufman, M. (1979). Cephalic neurulation and optic vesicle formation in the early mouse embryo. Am J Anat 155, 425–443. Abstract DOI
- Kawakami, K. Sato, S. Ozaki, H. Ikeda, K. (2000). Six family genes–structure and function as transcription factors and their roles in development. Bioessays 22, 616–626. Abstract Abstract
- Knecht, A.K. Bronner-Fraser, M. (2002). Induction of the neural crest: a multigene process. Nat Rev Genet 3, 453–461. Abstract DOI
- Knight, R.D. Nair, S. Nelson, S.S. Afshar, A. Javidan, Y. Geisler, R. Rauch, G.J. Schilling, T.F. (2003). lockjaw encodes a zebrafish tfap2a required for early neural crest development. Development 130, 5755–5768. Abstract DOI
- Kobayashi, M. Osanai, H. Kawakami, K. Yamamoto, M. (2000). Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech Dev 98, 151–155. Abstract DOI
- Kozlowski, D.J. Murakami, T. Ho, R.K. Weinberg, E.S. (1997). Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem Cell Biol 75, 551–562. Abstract DOI
- Kozlowski, D.J. Whitfield, T.T. Hukriede, N.A. Lam, W.K. Weinberg, E.S. (2005). The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol 277, 27–41. Abstract DOI
- Kroll, K.L. Salic, A.N. Evans, L.M. Kirschner, M.W. (1998). Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development 125, 3247–3258. Abstract Abstract
- Kudoh, T. Concha, M.L. Houart, C. Dawid, I.B. Wilson, S.W. (2004). Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development 131, 3581–3592. Abstract DOI
- Kumar, J.P. Moses, K. (2001). Eye specification in Drosophila: perspectives and implications. Semin Cell Dev Biol 12, 469–474. Abstract DOI
- LaBonne, C. Bronner-Fraser, M. (1998). Neural crest induction in Xenopus: evidence for a two-signal model. Development 125, 2403–2414. Abstract Abstract
- LaMantia, A.S. Bhasin, N. Rhodes, K. Heemskerk, J. (2000). Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron 28, 411–425. Abstract DOI
- Laclef, C. Souil, E. Demignon, J. Maire, P. (2003). Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev 120, 669–679. Abstract DOI
- Ladher, R.K. Anakwe, K.U. Gurney, A.L. Schoenwolf, G.C. Francis-West, P.H. (2000). Identification of synergistic signals initiating inner ear development. Science 290, 1965–1968. Abstract DOI
- Lang, R.A. (2004). Pathways regulating lens induction in the mouse. Int J Dev Biol 48, 783–791. Abstract DOI
- Lassiter, R.N. Dude, C.M. Reynolds, S.B. Winters, N.I. Baker, C.V. Stark, M.R. (2007). Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Dev Biol 308, 392–406. Abstract DOI
- Lee, S.A. Shen, E.L. Fiser, A. Sali, A. Guo, S. (2003). The zebrafish forkhead transcription factor Foxi1 specifies epibranchial placode-derived sensory neurons. Development 130, 2669–2679. Abstract DOI
- Leger, S. Brand, M. (2002). Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev 119, 91–108. Abstract DOI
- Li, X. Oghi, K.A. Zhang, J. Krones, A. Bush, K.T. Glass, C.K. Nigam, S.K. Aggarwal, A.K. Maas, R. Rose, D.W. et al. (2003). Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 426, 247–254. Abstract DOI
- Linker, C. Stern, C.D. (2004). Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development 131, 5671–5681. Abstract DOI
- Litsiou, A. Hanson, S. Streit, A. (2005). A balance of FGF, Wnt and BMP signalling positions the future placode territory in the head. Development 132, 4051–4062. Abstract DOI
- Liu, D. Chu, H. Maves, L. Yan, Y.L. Morcos, P.A. Postlethwait, J.H. Westerfield, M. (2003). Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development 130, 2213–2224. Abstract DOI
- Lopez-Rios, J. Tessmar, K. Loosli, F. Wittbrodt, J. Bovolenta, P. (2003). Six3 and Six6 activity is modulated by members of the groucho family 10.1242/dev.00185. Development 130, 185–195. Abstract DOI
- Luo, T. Matsuo-Takasaki, M. Lim, J.H. Sargent, T.D. (2001). Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int J Dev Biol 45, 681–684. Abstract Abstract
- Luo, T. Lee, Y.-H. Saint-Jeannet, J.-P. Sargent, T.D. (2003). Induction of neural crest in Xenopus by transcription factor AP2α10.1073/pnas.0237226100. Proc Natl Acad Sci USA 100, 532–537. Abstract DOI
- Marchant, L. Linker, C. Ruiz, P. Guerrero, N. Mayor, R. (1998). The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol 198, 319–329. Abstract Abstract
- Mardon, G. Solomon, N.M. Rubin, G.M. (1994). dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473–3486. Abstract Abstract
- Maroon, H. Walshe, J. Mahmood, R. Kiefer, P. Dickson, C. Mason, I. (2002). Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development 129, 2099–2108. Abstract Abstract
- Martin, K. Groves, A.K. (2006). Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development 133, 877–887. Abstract DOI
- Matsuo-Takasaki, M. Matsumura, M. Sasai, Y. (2005). An essential role of Xenopus Foxi1a for ventral specification of the cephalic ectoderm during gastrulation. Development 132, 3885–3894. Abstract DOI
- Mayor, R. Guerrero, N. Martinez, C. (1997). Role of FGF and noggin in neural crest induction. Dev Biol 189, 1–12. Abstract DOI
- McCabe, K.L. Bronner-Fraser, M. (2008). Essential role for PDGF signaling in ophthalmic trigeminal placode induction 10.1242/dev.017954. Development 135, 1863–1874. Abstract DOI
- McLarren, K.W. Litsiou, A. Streit, A. (2003). DLX5 positions the neural crest and preplacode region at the border of the neural plate. Dev Biol 259, 34–47. Abstract DOI
- Mishima, N. Tomarev, S. (1998). Chicken Eyes absent 2 gene: isolation and expression pattern during development. Int J Dev Biol 42, 1109–1115. Abstract Abstract
- Mizuseki, K. Kishi, M. Shiota, K. Nakanishi, S. Sasai, Y. (1998). SoxD: an essential mediator of induction of anterior neural tissues in Xenopus embryos. Neuron 21, 77–85. Abstract DOI
- Monsoro-Burq, A.H. Fletcher, R.B. Harland, R.M. (2003). Neural crest induction by paraxial mesoderm in Xenopus embryos requires FGF signals. Development 130, 3111–3124. Abstract DOI
- Moser, M. Imhof, A. Pscherer, A. Bauer, R. Amselgruber, W. Sinowatz, F. Hofstadter, F. Schule, R. Buettner, R. (1995). Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development 121, 2779–2788. Abstract Abstract
- Mulrenin, E.M. Witkin, J.W. Silverman, A.J. (1999). Embryonic development of the gonadotropin-releasing hormone (GnRH) system in the chick: a spatio-temporal analysis of GnRH neuronal generation, site of origin, and migration. Endocrinology 140, 422–433. Abstract DOI
- Murakami, S. Arai, Y. (1994). Transient expression of somatostatin immunoreactivity in the olfactory-forebrain region in the chick embryo. Brain Res Dev Brain Res 82, 277–285. Abstract DOI
- Neave, B. Holder, N. Patient, R. (1997). A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech Dev 62, 183–195. Abstract DOI
- Nechiporuk, A. Linbo, T. Raible, D.W. (2005). Endoderm-derived Fgf3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish 10.1242/dev.01876. Development 132, 3717–3730. Abstract DOI
- Nechiporuk, A. Linbo, T. Poss, K.D. Raible, D.W. (2007). Specification of epibranchial placodes in zebrafish. Development 134, 611–623. Abstract DOI
- Nguyen, V.H. Schmid, B. Trout, J. Connors, S.A. Ekker, M. Mullins, M.C. (1998). Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 199, 93–110. Abstract DOI
- Nieto, M.A. Sargent, M.G. Wilkinson, D.G. Cooke, J. (1994). Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264, 835–839. Abstract DOI
- Nieuwkoop, P. Faber, J. (1994). Normal Table of Xenopus Laevis (daudin) (2nd edn). 2nd edn. New York & London: Garland Publishing;
- Nikaido, M. Doi, K. Shimizu, T. Hibi, M. Kikuchi, Y. Yamasu, K. (2007). Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn 236, 564–571. Abstract DOI
- Nissen, R.M. Yan, J. Amsterdam, A. Hopkins, N. Burgess, S.M. (2003). Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development 130, 2543–2554. Abstract DOI
- Niswander, L. Martin, G.R. (1992). Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 114, 755–768. Abstract Abstract
- Ohto, H. Kamada, S. Tago, K. Tominaga, S.I. Ozaki, H. Sato, S. Kawakami, K. (1999). Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol 19, 6815–6824. Abstract Abstract
- Ohyama, T. Groves, A.K. (2004). Expression of mouse Foxi class genes in early craniofacial development. Dev Dyn 231, 640–646. Abstract DOI
- Ohyama, T. Mohamed, O.A. Taketo, M.M. Dufort, D. Groves, A.K. (2006). Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865–875. Abstract DOI
- Ohyama, T. Groves, A.K. Martin, K. (2007). The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol 51, 463–472. Abstract DOI
- Ozaki, H. Nakamura, K. Funahashi, J. Ikeda, K. Yamada, G. Tokano, H. Okamura, H.O. Kitamura, K. Muto, S. Kotaki, H. et al. (2004). Six1 controls patterning of the mouse otic vesicle. Development 131, 551–562. Abstract DOI
- Pandur, P.D. Moody, S.A. (2000). Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in the differentiating lateral lines. Mech Dev 96, 253–257. Abstract DOI
- Papalopulu, N. Kintner, C. (1993). Xenopus Distal-less related homeobox genes are expressed in the developing forebrain and are induced by planar signals. Development 117, 961–975. Abstract Abstract
- Papanayotou, C. Mey, A. Birot, A.M. Saka, Y. Boast, S. Smith, J.C. Samarut, J. Stern, C.D. (2008). A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS Biol 6, e2. Abstract DOI
- Pappu, K.S. Mardon, G. (2004). Genetic control of retinal specification and determination in Drosophila. Int J Dev Biol 48, 913–924. Abstract DOI
- Penzel, R. Oschwald, R. Chen, Y. Tacke, L. Grunz, H. (1997). Characterization and early embryonic expression of a neural specific transcription factor xSOX3 in Xenopus laevis. Int J Dev Biol 41, 667–677. Abstract Abstract
- Pera, E. Stein, S. Kessel, M. (1999). Ectodermal patterning in the avian embryo: epidermis versus neural plate. Development 126, 63–73. Abstract Abstract
- Phillips, B.T. Bolding, K. Riley, B.B. (2001). Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol 235, 351–365. Abstract DOI
- Pignoni, F. Hu, B. Zavitz, K.H. Xiao, J. Garrity, P.A. Zipursky, S.L. (1997). The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development [published erratum appears in Cell 1998 Feb 20;92(4):following 585]. Cell 91, 881–891. Abstract DOI
- Quiring, R. Walldorf, U. Kloter, U. Gehring, W.J. (1994). Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265, 785–789. Abstract DOI
- Rayapureddi, J.P. Kattamuri, C. Steinmetz, B.D. Frankfort, B.J. Ostrin, E.J. Mardon, G. Hegde, R.S. (2003). Eyes absent represents a class of protein tyrosine phosphatases. Nature 426, 295–298. Abstract DOI
- Rebay, I. Silver, S.J. Tootle, T.L. (2005). New vision from Eyes absent: transcription factors as enzymes. Trends Genet 21, 163–171. Abstract DOI
- Rex, M. Orme, A. Uwanogho, D. Tointon, K. Wigmore, P.M. Sharpe, P.T. Scotting, P.J. (1997). Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn 209, 323–332. Abstract Abstract
- Riley, B.B. Phillips, B.T. (2003). Ringing in the new ear: resolution of cell interactions in otic development. Dev Biol 261, 289–312. Abstract DOI
- Ruf, R.G. Xu, P.X. Silvius, D. Otto, E.A. Beekmann, F. Muerb, U.T. Kumar, S. Neuhaus, T.J. Kemper, M.J. Raymond, R.M. et al. (2004). SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci USA 101, 8090–8095. Abstract DOI
- Sasai, Y. De Robertis, E.M. (1997). Ectodermal patterning in vertebrate embryos. Dev Biol 182 , 5–20. Article DOI
- Schlosser, G. Northcutt, R.G. (2000). Development of neurogenic placodes in Xenopus laevis. J Comp Neurol 418, 121–146. Abstract Abstract
- Schlosser, G. Ahrens, K. (2004). Molecular anatomy of placode development in Xenopus laevis. Dev Biol 271, 439–466. Abstract DOI
- Schlosser, G. (2006). Induction and specification of cranial placodes. Dev Biol 294, 303–351. Abstract DOI
- Schwob, J.E. (2002). Neural regeneration and the peripheral olfactory system. Anat Rec 269, 33–49. Abstract DOI
- Serikaku, M.A. O’Tousa, J.E. (1994). sine oculis is a homeobox gene required for Drosophila visual system development. Genetics 138, 1137–1150. Abstract Abstract
- Shamim, H. Mason, I. (1999). Expression of Fgf4 during early development of the chick embryo. Mech Dev 85, 189–192. Abstract DOI
- Shen, W. Mardon, G. (1997). Ectopic eye development in Drosophila induced by directed dachshund expression. Development 124, 45–52. Abstract Abstract
- Sheng, G. Stern, C.D. (1999). Gata2 and Gata3: novel markers for early embryonic polarity and for non-neural ectoderm in the chick embryo. Mech Dev 87, 213–216. Abstract DOI
- Sheng, G. dos Reis, M. Stern, C.D. (2003). Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell 115, 603–613. Abstract DOI
- Sjodal, M. Edlund, T. Gunhaga, L. (2007). Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell 13, 141–149. Abstract DOI
- Solomon, K.S. Fritz, A. (2002). Concerted action of two dlx paralogs in sensory placode formation. Development 129, 3127–3136. Abstract Abstract
- Solomon, K.S. Kudoh, T. Dawid, I.B. Fritz, A. (2003). Zebrafish foxi1 mediates otic placode formation and jaw development. Development 130, 929–940. Abstract DOI
- Stern, C.D. (2005). Neural induction: old problem, new findings, yet more questions. Development 132, 2007–2021. Abstract DOI
- Streit, A. Lee, K.J. Woo, I. Roberts, C. Jessell, T.M. Stern, C.D. (1998). Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development 125, 507–519. Abstract Abstract
- Streit, A. Stern, C.D. (1999). Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mech Dev 82, 51–66. Abstract DOI
- Streit, A. Berliner, A.J. Papanayotou, C. Sirulnik, A. Stern, C.D. (2000). Initiation of neural induction by FGF signalling before gastrulation. Nature 406, 74–78. Abstract DOI
- Streit, A. (2002). Extensive cell movements accompany formation of the otic placode. Dev Biol 249, 237–254. Abstract DOI
- Streit, A. (2007). The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol 51, 447–461. Abstract DOI
- Sun, S.K. Dee, C.T. Tripathi, V.B. Rengifo, A. Hirst, C.S. Scotting, P.J. (2007). Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol 303, 675–686. Abstract DOI
- Suzuki, A. Ueno, N. Hemmati-Brivanlou, A. (1997). Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 124, 3037–3044. Abstract Abstract
- Toba, Y. Ajiki, K. Horie, M. Sango, K. Kawano, H. (2001). Immunohistochemical localization of calbindin D-28k in the migratory pathway from the rat olfactory placode. J Neuroendocrinol 13, 683–694. Abstract DOI
- Tribulo, C. Aybar, M.J. Nguyen, V.H. Mullins, M.C. Mayor, R. (2003). Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development 130, 6441–6452. Abstract DOI
- Vendrell, V. Carnicero, E. Giraldez, F. Alonso, M.T. Schimmang, T. (2000). Induction of inner ear fate by FGF3. Development 127, 2011–2019. Abstract Abstract
- Vonica, A. Brivanlou, A.H. (2006). An obligatory caravanserai stop on the silk road to neural induction: inhibition of BMP/GDF signaling. Semin Cell Dev Biol 17, 117–132. Abstract DOI
- Waddington, C.H. (1937). The determination of the auditory placode in the chick. J Exp Biol 14, 232–239.
- Wawersik, S. Purcell, P. Rauchman, M. Dudley, A.T. Robertson, E.J. Maas, R. (1999). BMP7 acts in murine lens placode development. Dev Biol 207, 176–188. Abstract DOI
- Wawersik, S. Maas, R.L. (2000). Vertebrate eye development as modeled in Drosophila. Hum Mol Genet 9, 917–925. Abstract DOI
- Wawersik, S. Evola, C. Whitman, M. (2005). Conditional BMP inhibition in Xenopus reveals stage-specific roles for BMPs in neural and neural crest induction. Dev Biol 277, 425–442. Abstract DOI
- Weasner, B. Salzer, C. Kumar, J.P. (2007). Sine oculis, a member of the SIX family of transcription factors, directs eye formation. Dev Biol 303, 756–771. Abstract DOI
- Webb, . (1993). Ectodermal placodes: contributions to the development of the vertebrate head. Am Zool 33, 434–447.
- Whitfield, T.T. (2005). Lateral line: precocious phenotypes and planar polarity. Curr Biol 15, R67–70. Article DOI
- Wilson, P.A. Lagna, G. Suzuki, A. Hemmati-Brivanlou, A. (1997). Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development 124, 3177–3184. Abstract Abstract
- Wilson, S.I. Graziano, E. Harland, R. Jessell, T.M. Edlund, T. (2000). An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol 10, 421–429. Abstract DOI
- Woda, J.M. Pastagia, J. Mercola, M. Artinger, K.B. (2003). Dlx proteins position the neural plate border and determine adjacent cell fates. Development 130, 331–342. Abstract DOI
- Wray, S. (2002). Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol 23, 292–316. Abstract DOI
- Wright, T.J. Mansour, S.L. (2003). Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130, 3379–3390. Abstract DOI
- Xu, P.X. Adams, J. Peters, H. Brown, M.C. Heaney, S. Maas, R. (1999). Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23, 113–117. Abstract DOI
- Xu, H. Dude, C.M. Baker, C.V.H. (2008). Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev Biol 317, 174–186. Abstract DOI
- Zelarayan, L.C. Vendrell, V. Alvarez, Y. Domínguez-Frutos, E. Theil, T. Alonso, M.T. Maconochie, M. Schimmang, T. (2007). Differential requirements for FGF3, FGF8 and FGF10 during inner ear development. Dev Biol 308, 379–391. Abstract DOI
- Zheng, W. Huang, L. Wei, Z.B. Silvius, D. Tang, B. Xu, P.X. (2003). The role of Six1 in mammalian auditory system development. Development 130, 3989–4000. Abstract DOI
- Zhu, C.C. Dyer, M.A. Uchikawa, M. Kondoh, H. Lagutin, O.V. Oliver, G. (2002). Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development 129, 2835–2849. Abstract Abstract
- Zou, D. Silvius, D. Fritzsch, B. Xu, P.X. (2004). Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development 131, 5561–5572. Abstract DOI
- Zou, D. Silvius, D. Rodrigo-Blomqvist, S. Enerback, S. Xu, P.-X. (2006). Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Dev Biol 298, 430–441. Abstract DOI
- von Woellwarth, C. (1961). Die Rolle des Neuralleistenmaterials und der Temperatur bei der Determination der Augenlinse. Embryologia 6, 219–242. Article DOI