The cornea on the front surface of the eye is our window to the world, hence maintenance of corneal tissue transparency is essential for vision. The integrity and functionality of the outermost corneal layer, the epithelium, plays a key role in refraction of light on to the retina at the back of the eye. Like other epithelia, the epithelium of the cornea is maintained by stem cells. This review will discuss what is currently known about the properties of these stem cells, the clinical consequences of stem cell failure and the potential for stem cell therapy in regeneration of the ocular surface.
1. Function and structure of the cornea
The cornea is responsible for protecting the eye against insults such as injury and infection. It also provides the majority (two thirds) of the total refractive power of the eye and is therefore the major refracting lens (Meek et al., (Meek et al., 2003)).
The cornea is comprised of five layers (see Figure 1), the outermost non-keratinised stratified epithelium, Bowman's layer, a highly ordered keratocyte-populated collagenous stroma, Descemet's membrane and the inner endothelium (a cellular monolayer).
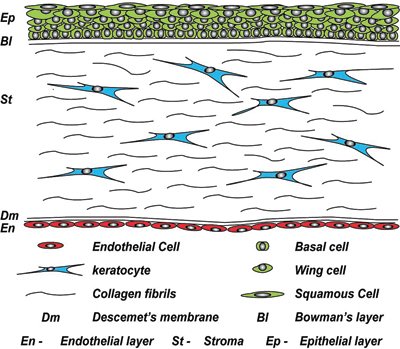
Figure 1. The human cornea in cross-section. At the outer surface of the cornea, there is an epithelial layer, which sits on a basement membrane above Bowman's layer. The middle stromal layer, which is sparsely populated with keratocytes is surrounded by dense connective tissue. The final layer consists of a single sheet of endothelial cells, which sits on Descemet's membrane.
2. Corneal development
Development of the anterior chamber of the eye (comprised of the cornea, lens, ciliary body, iris, trabecular meshwork and aqueous humour) requires the interaction of cells from the surface epithelium and neuroepithelium with mesenchymal cells predominantly of neural crest origin.
Anterior eye development first begins with the formation of the lens placode. This forms after the optic vesicles come in contact with the surface ectoderm. A thickening forms, that enlarges and forms a lens pit. Between days E8.5 and 9.5 in mouse this lens pit becomes the lens vesicle and remains connected to the surface ectoderm via a lens stalk (Kaufman, (1992); Pei and Rhodin, (1970)). Eventually this lens vesicle detaches from the surface ectoderm and invaginates into the optic cup. Shortly after this detachment, periocular mesenchymal cells derived from somitomeric mesoderm and forebrain neural crest migrate into the space between the anterior lens vesicle epithelium and the surface ectoderm, eventually forming keratocytes and corneal endothelium (Trainor and Tam, (1995)).
In mice, four to seven layers of mesenchymal cells are seen at E12. These cells have long cytoplasmic extensions with a star shaped phenotype (Haustein, (1983)). Cell numbers continue to increase and condense to form several layers of separated flattened cells. At E14.5 to E15.5 the cells adjacent to the lens structure form the endothelium (Reneker et al., (Reneker et al., 2000)). The surface ectoderm cells overlaying the mesenchymal cells become the corneal epithelium. The remaining mesenchymal cells between these two layers differentiate into corneal stromal fibroblasts (Cintron et al., (Cintron et al., 1983)). This differs with humans, where there is a second wave of mesenchymal cell migration into the space between the newly formed endothelial layer and the surface ectoderm. These cells differentiate into corneal fibroblasts. In mouse the proliferative potential of corneal fibroblasts diminishes during development from birth to eyelid opening, however they arrest in the G0 phase of the cell cycle as opposed to becoming terminally differentiated (Zieske et al., (Zieske et al., 2001)). As the corneal endothelium differentiates, the lens detaches from the immature corneal structure. This allows the formation of a fluid filled area in to which the iris and ciliary body grow.
Studies resulting in abnormal corneal development due to the over expression of growth factors such as TGFα, FGF3 and EGF in the lens, highlight the importance of the lens in cornea development (Coulombre and Coulombre, (1964); Reneker et al., (Reneker et al., 1995); Reneker et al., (Reneker et al., 2000); Robinson et al., (Robinson et al., 1998)). The ectoderm overlaying the lens becomes the corneal epithelium. In its primitive state the epithelium is 1–2 cell layers thick and later stratifies to three to four cell layers following lens detachment. The eyelids then form and fuse with the primitive epithelium being reduced to 1–2 cells layers thick until eyelid opening which occurs at 24 weeks gestation in humans and P12 to P14 in mice.
For up to seven days of age the corneal and limbal epithelia in rats is 1–2 cells layers (Chung et al., (Chung et al., 1992)). Prior to eyelid opening at 10 days the epithelial thickness increases to two to three layers. Further increases to four-five cell layers occur in the central cornea following eyelid opening at two weeks of age (Chung et al., (Chung et al., 1992); Watanabe et al., (Watanabe et al., 1993)). The layers continue to increase until four weeks of age when the epithelium reaches adult levels of six to seven cell layers (Song et al., (Song et al., 2003)). The basal epithelial cell shape also changes with development. Initially the cells are flat and ovoid in shape until eyelid opening after which they become more cuboidal. By three weeks the basal cells are more columnar in the central cornea but not the limbal region (Chung et al., (Chung et al., 1992)).
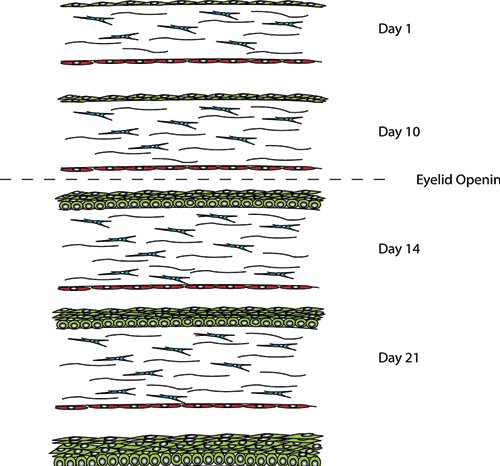
Figure 2. Development of the rodent cornea. Between days P1 and P7 the epithelial layer is 1-2 cell layers thick until just prior to eyelid opening at day P10, when this increases to 2-3 cell layers. Following eyelid opening at day P14 the number cell layers increases to 4-5 with 5-6 cells layers being present at 3 weeks of age. At P28 the corneal epithelium is representative of the adult epithelium, with a single layer of columnar basal cells, which become flattened as they move to the surface.
3. The stroma and endothelium
The stroma is a mesenchymal tissue derived from the neural crest. The dense tissue of the stroma accounts for 90% of the total corneal thickness. The parallel arrangement of lamellae formed from heterodimeric complexes of type I and type V collagen fibres maintain transparency (Fini and Stramer, (2005)). These collagen fibres are held in a uniform spacing pattern by proteoglycans. Keratocytes (fibroblasts) are located between the lamellae (Hay et al., (Hay et al., 1979)). These sparsely located keratocytes link to one another via dendritic processes (Muller et al., (Muller et al., 1995)) and produce crystalline proteins to maintain corneal transparency (Jester et al., (Jester et al., 1999)). Recent reports have described a keratocyte stem cell population in the anterior stroma (Du et al., (Du et al., 2005); Funderburgh et al., (Funderburgh et al., 2005)).
Descemet's membrane rests on the innermost surface of the cornea. It acts as a basement membrane for the inner endothelial cell monolayer. These cells transport nutrients from the aqueous humour to the stroma and concurrently pump out excess water preventing corneal oedema (swelling) by maintaining optimal hydration.
4. The corneal epithelium
The corneal epithelium is a dynamic physical barrier preventing the entry of deleterious agents into the intraocular space. It consists of superficial squamous cells, central suprabasal cells and a single layer of inner columnar basal cells. The differentiated squamous cells have surface microvilli and occupy the outer 1–3 cell layers of the epithelium. The function of the microvilli is to increase cell surface area allowing close association with the tear film. Highly resistant tight junctions formed between neighbouring cells provide a protective barrier (Klyce, (1972)). The underlying suprabasal cells have wing-like extensions, rarely undergo division and migrate superficially to differentiate into squamous cells.
The inner basal cells consist of a single layer of columnar cells with several important functions including the generation of new suprabasal cells. Additionally, they secrete matrix factors important for basement membrane and stromal function. The basal cells also regulate organisation of hemidesmonsomes and focal complexes to maintain attachment to the underlying basement membrane. These functions are suggested to be important in mediating cell migration in response to epithelial injury (Pajoohesh-Ganji and Stepp, (2005)).
5. Homeostasis in the corneal epithelium
Corneal integrity and therefore function is dependent upon the self-renewing properties of the corneal epithelium. The prevailing hypothesis is that this renewal relies on a small population of putative stem cells located in the basal region of the limbus. These putative stem cells are primitive and can divide symmetrically to self renew and asymmetrically to produce daughter transit amplifying cells (TAC) that migrate centripetally to populate the basal layer of the corneal epithelium (see Figure 3; Kinoshita et al., (Kinoshita et al., 1981); Tseng, (1989)). The TAC divide and migrate superficially, progressively becoming more differentiated, eventually becoming post-mitotic terminally differentiated (TD) cells. Using suppressive subtractive hybridisation, Sun et al., (Sun et al., 2006) identified a novel gene (EEDA) with localisation to corneal basal and suprabasal cells, suggesting it is involved in early stage stratification of epithelial differentiation (Sun et al., (Sun et al., 2006)).
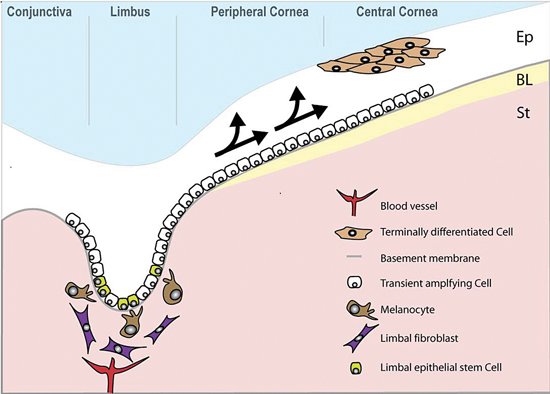
Figure 3. The human limbus. Limbal epithelial stem cells reside in the basal layer of the epithelium (Ep), which undulates at the limbus. Daughter transient amplifying cells (TACs) divide and migrate towards the central cornea (arrowed) to replenish the epithelium, which rests on Bowman's layer (BL). The stroma (St) of the limbal epithelial stem cell niche is populated with fibroblasts and melanocytes and also has a blood supply.
Once fully differentiated TD squamous cells are shed from the ocular surface during normal wear and tear and this in turn stimulates the cycle of cell division, migration and differentiation (Beebe and Masters, (1996)). Thoft and Friend developed the ‘The X, Y, Z hypothesis of corneal epithelial maintenance’. This hypothesis proposed that the addition of the proliferation of basal cells (X) and the centripetal migration of cells (Y) was equal to epithelial cell loss from the corneal surface. However, they were unable to rule out the involvement of the neighbouring bulbar conjunctiva (Thoft and Friend, (1983)). Later, mathematical analysis indicated that the corneal epithelial cell mass could be renewed by cells from the limbal epithelium alone (Sharma and Coles, (1989)). Furthermore, a fine balance between cell proliferation, differentiation, migration and apoptosis is necessary. A variety of cytokines have been shown to play important roles in the maintenance and wound healing of the cornea. These factors are supplied in part by the adjacent tear film and the aqueous humour (Welge-Lussen et al., (Welge-Lussen et al., 2001)). Other growth factors are produced by keratocytes in the supporting stroma (West-Mays and Dwivedi, (2006)) and by the corneal epithelial cells themselves (Rolando and Zierhut, (2001)).
6. Limbal epithelial stem cells
Throughout life, our self-renewing tissues rely upon populations of stem cells / progenitors to replenish themselves throughout life following normal wear and tear and injury. The corneal epithelium on the front surface of the eye is no exception as dead squamous cells are constantly sloughed from the corneal epithelium during blinking. At the corneo-scleral junction in an area known as the limbus, there is a population of limbal epithelial stem cells (LESCs). LESCs share common features with other adult somatic stem cells including small size (Romano et al., (Romano et al., 2003)) and high nuclear to cytoplasmic ratio (Barrandon and Green, (1987)). They also lack expression of differentiation markers such as cytokeratins 3 and 12 (Kurpakus et al., (Kurpakus et al., 1990); Schermer et al., (Schermer et al., 1986)).
LESCs are slow cycling during homeostasis and therefore retain DNA labels for long time periods, however in the event of injury they can become highly proliferative (Cotsarelis et al., (Cotsarelis et al., 1989); Lavker and Sun, (2003); Lehrer et al., (Lehrer et al., 1998)). To replenish the stem cell pool, stem cells have the ability to divide asymmetrically (see Figure 4).
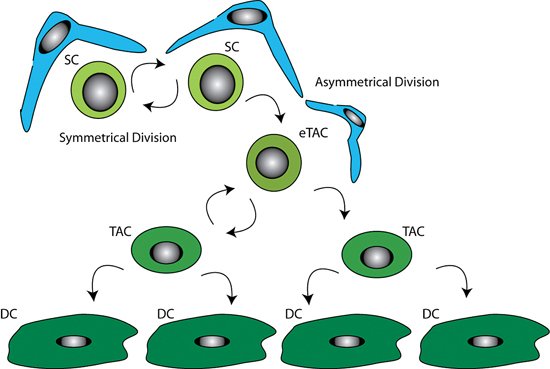
Figure 4. Illustration of stem cell control by the niche. It is thought LESC undergo asymmetric cell division producing a stem cell, which remains in the stem cell niche to repopulate the stem cell pool, and a daughter early transient amplifying cell (eTAC). This more differentiated eTAC is removed from the stem cell niche and is able to divide further producing transient amplifying cells (TAC), eventually giving rise to terminally differentiated cells (DC). The double arrows represent the self-renewing capability of the stem cells. The supporting niche cells (blue) surround the stem cells (light green).
Expression of C/EBPΔ in a subset of LESC both in vivo and in vitro has recently been suggested to be involved in the regulation of self-renewal and LESC cell cycle length (Barbaro et al., (Barbaro et al., 2007)).
7. Evidence for stem cells in the corneal limbus
The first experimental indication of the presence of stem cells in the limbus was the observation of pigment (melanin) movement from the limbus to towards an epithelial defect following wounding of rabbit corneas (Mann, (1944)).
Davanger and Evenson later observed a similar centripetal migration of pigment from limbus to central cornea in humans. Hence they proposed that the limbal Palisades of Vogt (PV) were the source of LESC (Davanger and Evenson, (1971); Huang and Tseng, (1991)). Following lamellar keratoplasty, this centripetal migration was also observed in the rabbit as host epithelium was gradually replaced with donor epithelium (Kinoshita et al., (Kinoshita et al., 1981)). Furthermore, the complete removal of the limbus results in impaired corneal function, neovascularisation and conjunctival ingrowth (Huang and Tseng, (1991)).
Stem cells may be identified by the retention of DNA labels as they are slow cycling and only divide occasionally (Bickenbach, (1981)). Assuming stem cell division during the labelling period, stem cell exposure to DNA precursors such as tritiated thymidine or bromodeoxyuridine followed by chase periods of up to 8 weeks labels the slow cycling cells (presumed to be stem cells). The more differentiated and more rapidly dividing daughter transit amplifying cells (TAC) undergo dilution of the label through multiple divisions. Through the use of tritiated thymidine, Cotsarelis et al, found slow cycling label retaining cells (LRCs) in the limbal basal epithelial region of the mouse cornea and postulated that up to 10% of limbal basal cells were stem cells (Cotsarelis et al., (Cotsarelis et al., 1989)). Phenotypically this population of cells appear to be more primitive in nature as they remain small and round (Romano et al., (Romano et al., 2003)).
Limbal basal cells exhibit higher proliferative potential when compared to peripheral and central cornea both in vitro and in vivo. Large epithelial wounds in rabbits heal faster than smaller central defects. This implies that the proliferative capacity of the peripheral cornea is greater than that of the central (Lavker et al., (Lavker et al., 1991)). In the human, limbal explant cultures have greater proliferative potential when compared to central explants (Ebato et al., (Ebato et al., 1987); Ebato et al., (Ebato et al., 1988)). Furthermore, LESC proliferation is resistant to inhibition by tumour-promoting phorbol esters (Kruse and Tseng, (1993); Lavker et al., (Lavker et al., 1998)). Based upon the methods of characterisation used to identify features of stem cells isolated and cultured from human epidermis (Barrandon and Green, (1987)), similar clonogenicity studies on cells isolated from the limbus produced large holoclone colonies (stem cell derived) with extended cell generation number. The less clonogenic meroclones and paraclones were found elsewhere in the cornea (Pellegrini et al., (Pellegrini et al., 1999)).
Clinical evidence also points toward the limbus as a depository for a stem cell population. During homeostasis, the limbal epithelial cells are thought to act as a barrier preventing conjunctival epithelial cells from encroaching upon the cornea (Tseng, (1989)). During LESC failure (to be discussed later), the conjunctiva can invade the cornea causing chronic inflammation, painful corneal opacity and neovascularisation. Ambati et al., have recently shown experimentally that soluble vascular endothelial growth factor receptor 1 (sFlt1) is important for corneal avascularity (Ambati et al., (Ambati et al., 2006)). They have since found expression of sFlt1 in normal human corneal epithelium and a reduction of sFlt1 in vascularised patients (Ambati et al., (Ambati et al., 2007)). Further clinical evidence pointing to the location of LESC at the limbus was demonstrated by Kenyon and Tseng, who transplanted two limbal explants taken from the contralateral healthy eye of patients on the damaged eye. This resulted in re-epithelisation of the cornea and regression of persistent epithelial defects and neovascularisation (Kenyon and Tseng, (1989)).
The dogma that stem cells which give rise to corneal epithelial cells exclusively reside in the limbus was recently challenged. In the mouse it was demonstrated that central corneal epithelium could be serially transplanted and that it contains oligopotent stem cells that can maintain the corneal epithelium without cellular input from the limbal region. Furthermore, holoclone colonies were cultured from the central corneas of a number of mammalian species including from two human donors (Majo et al., (Majo et al., 2008)). However, both human donors were 4 years or younger so it will be interesting to see if the results are reproducible in the adult human cornea when development of the eye is complete.
In the skin, the existence of transit amplifying cells has also been questioned. Rather than stem cells producing transit amplifying cells to maintain homeostasis in the epidermis, it has been proposed that a population of ‘committed progenitor’ cells fulfil this function during normal tissue turn over. It is proposed that the stem cells are only called into action in response to injury (Clayton et al., (Clayton et al., 2007); Jones et al., (Jones et al., 2007)). Similarly, it has been proposed that function of LESCs is to respond to injury and not to look after normal wear and tear of the corneal epithelium (Majo et al., (Majo et al., 2008)). It remains to be determined if the long-accepted transit amplifying cell hypothesis continues to hold true for the corneal epithelium.
7.1. The LESC niche
The stem cell niche, or microenvironment consisting of cellular and extracellular components, is hypothesised to prevent stem cell differentiation and thus regulates their fate (Schofield, (1983); Watt and Hogan, (2000)). When a stem cell divides asymmetrically, one daughter may leave the niche to enter a differentiation pathway under the influence of different environmental stimuli. The limbus differs from cornea both anatomically and functionally and hence could differentially determine stem cell fate.
Within the limbal region of the cornea, the LESC niche is thought to be located within the palisades of Vogt (PV) – an undulating region of increased surface area. The palisades are highly pigmented with melanocytes (Davanger and Evenson, (1971); Higa et al., (Higa et al., 2005)) and are infiltrated with Langerhan's cells (Baum, (1970)) and T-lymphocytes (Vantrappen et al., (Vantrappen et al., 1985)). The melanin pigmentation is thought to shield LESCs from damaging ultraviolet light and the resultant generation of reactive oxygen species (Shimmura and Tsubota, (1997)). The deep undulations of the Palisades of Vogt at the limbus provide LESC with an environment that protect them from shearing forces (Gipson, (1989)). Furthermore the crypts described by Shortt et al., predominantly occur on the superior and inferior cornea where they are normally covered by the eye lids. (Shortt et al., (Shortt et al., 1402)) This may reflect the evolution of a protective environment for LESCs in humans. The basement membrane lining the LESC niche contains papillae of stroma that project upwards (Shortt et al., (Shortt et al., 1402)). The limbal and corneal basement membrane components also differ, with the limbal region containing laminin-1,5 and α2β2 chains not found in the cornea. Furthermore, type IV collagen α1, α2 and α5 chains are found in the limbal region whereas α3 and α5 are located in the cornea (Ljubimov et al., (Ljubimov et al., 1995); Tuori et al., 1996). A more recent study by Schlötzer-Schrehardt et al., found patchy immunolocalisation of laminin γ3 chain, BM40/SPARC and tenancin C, that was also found to co-localise with ABCG2/p63/K19-positive cell clusters. These factors may be involved in retaining cell stemness (Schlotzer-Schrehardt et al., (Schlotzer-Schrehardt et al., 2007)).
The basement membrane beneath the LESC may also act to sequester and therefore modulate growth factors and cytokines involved in LESC regulation and function (Klenkler and Sheardown, (2004)). Although the surface of the cornea is exposed to atmospheric oxygen, the LESC niche lies beneath a number of cell layers where the oxygen tension is likely to be lower. Interestingly, hypoxic in vitro conditions have been found to produce larger, less differentiated limbal epithelial cell colonies suggesting that low oxygen levels may induce selective proliferation of undifferentiated cells (Miyashita et al., (Miyashita et al., 2007)).
The limbal niche is vascularised and highly innervated (Lawrenson and Ruskell, (1991)) unlike the avascular cornea and therefore is a potential source of nutrients and growth factors for LESC. Limbal fibroblasts in the underlying stroma are heterogeneous and express secreted protein acidic and rich in cysteine (SPARC) that may contribute to LESC adhesion (Shimmura et al., (Shimmura et al., 2006)). Furthermore, Nakamura et al., identified a population of bone marrow-derived cells located in the limbal stroma following transplantation of GFP labelled bone marrow cells into nude mice (Nakamura et al., (Nakamura et al., 2005)). It is possible therefore that these cells are able to migrate into the limbal stroma, although any potential functionality remains unclear.
Sonic hedgehog, Wnt/β-catenin, TGF-β and Notch signalling pathways have all being implicated in niche control of stem cells, however little is known of their potential roles in the LESC niche. Mice lacking in expression of Dkk2, a Wnt pathway inhibitor, display epidermal differentiation on the ocular surface. The lack of Dkk2, leads to increased Wnt/β-catenin signalling in the limbal stroma. This demonstrates the importance of limbal niche control over LESC differentiation during development. PAX6 expression is also lost in the corneal epithelial cells of these mice, suggesting it is downstream of Dkk2 (Mukhopadhyay et al., (Mukhopadhyay et al., 2006)). Deficiencies in PAX6 leads to aniridia resulting in impaired corneal epithelial function and eventual LESC failure, which may be due to altered niche development.
7.2. Putative positive and negative LESC markers
The literature reflects many attempts to prospectively identify LESC using a specific marker. As yet no single, reliable marker has been found. However, the expression of a combination of several features seems to allow for greater specificity.
Putative ‘markers’ can either be positive (present) or negative (absent). Limbal basal cells lack differentiation markers such as the 64 kDa cytokeratin 3 (CK3) that is present in all other layers of the corneal epithelium and the suprabasal layers of the limbal epithelium (Schermer et al., (Schermer et al., 1986)). The corneal specific 55 kD protein, cytokeratin 12 (CK12) is also expressed in a similar pattern (Chaloin-Dufau et al., (Chaloin-Dufau et al., 1990)). Furthermore, connexin 43 (Shortt et al., (Shortt et al., 1402); Matic et al., (Matic et al., 1997)) and involucrin (Chen et al., (Chen et al., 2004)), both markers of cells destined for differentiation, are also absent.
The transcription factor p63 is required for formation of epidermis and has been proposed as a putative positive LESC marker (Pellegrini et al., (Pellegrini et al., 2001)). In vitro,p63 was found to be expressed in limbal epithelial cell derived holoclones with little or no expression in meroclones and paraclones. In vivo, p63 was located in the limbal basal epithelium. However, since these initial observations a number of reports have suggested that p63 is not sufficiently specific to act as an LESC marker as it has also been localised to basal cells of the peripheral and central cornea in humans (Chen et al., (Chen et al., 2004); Dua et al., (Dua et al., 2003)) and in rats (Chee et al., (Chee et al., 2006)). However, limbal epithelial cells expressing high levels of p63 with a high nuclear to cytoplasmic ratio appear to be more stem like (Arpitha et al., (Arpitha et al., 2005)). Further work has since indicated that the ΔNp63α isoform may more specifically label LESC (Di Iorio et al., (Di Iorio et al., 2005)).
Many types of organ-specific stem cells, including LESC have been recently shown to exhibit a side population (SP) phenotype. The SP cells are able to efflux Hoechst 33342 dye through the ATP-binding cassette transporter Bcrp1/ABCG2. ABCG2 has therefore been proposed to be a universal marker for stem cells (Zhou et al., (Zhou et al., 2001); Watanabe et al., (Watanabe et al., 2004)). In putative LESCs, this protein has been immunolocalised to the cell membrane and cytoplasm of a population of limbal basal cells and a few suprabasal cells (Chen et al., (Chen et al., 2004)). Furthermore, ABCG2 positive cells produce higher colony forming efficiency values in vitro than their negative counterparts (de Paiva et al., (de Paiva et al., 2005)). Our laboratory has localised ABCG2 to the outer edge of holoclones where it is thought that the stem cells reside.
Clusters of cells expressing the integrin α9 have been localised to the limbal basal epithelium (Stepp et al., (Stepp et al., 1995)). However, upregulation of α9 in wounded murine corneas have since indicated this integrin to be associated with TAC's (Stepp and Zhu, (1997)). Integrin β1 was originally suggested to be a keratinocyte marker (Jones and Watt, (1993)). Cells that rapidly adhere to the integrin β1 ligand, collagen IV also display LESC properties (Li and Lu, (2005)). Limbal basal epithelial cells are described as β1 integrin bright as are the stem cells of the epidermis suggesting a gradient of expression that decreases with differentiation. The integrins α2, α6 and β4 are negative in the limbal basal epithelial cells (Schlotzer-Schrehardt and Kruse, (2005)).
N-cadherin is an important mediator of cell-cell adhesion and may play a key role in the maintenance of haemopoietic stem cells by facilitating adhesion to osteoblasts in the bone marrow niche (Calvi et al., (Calvi et al., 2003); Zhang et al., (Zhang et al., 2003)). Hayashi et al found expression of N-cadherin in a subpopulation of limbal epithelial basal cells and in adjacent melancytes implying N-cadherin plays an important role in interactions between LESC and their corresponding niche cells (Hayashi et al., (Hayashi et al., 2007)).
Even though the limbal epithelium is derived from the surface ectoderm a number of neural stem cell markers have been suggested as LESC markers. Recent in depth immunological studies of neurotrophic factors and their receptors in the human has found NGF, glial cell-derived neurotrophic factor (GDNF) and their corresponding receptors TrkA and GDNF family receptor alpha (GFRα)-1 to be exclusively expressed in the limbus (Qi et al., (Qi et al., 2008)).
Notch 1 is a ligand-activated transmembrane receptor that has been shown to maintain progenitor cells in a number of tissues. The role of Notch signalling in the cornea is unclear. However, cell clusters in the palisades of Vogt have been found with some co-localisation with ABCG2 (Thomas et al., (Thomas et al., 2007)). Using Notch 1 deficient mice, Vauclair et al, demonstrated Notch 1 signalling is required for cell fate maintenance during corneal epithelial wound healing linking this to regulation of vitamin A metabolism (Vauclair et al., (Vauclair et al., 2007)). Notch 1, other Notch family members and their down-stream targets have been identified throughout the cornea suggesting a role in differentiation (Ma et al., (Ma et al., 2007)). More recently, Nakamura et al., has found Hes1, a major target in Notch1 signalling, to be localised to the basal limbal epithelium in adult mice (Nakamura et al., (Nakamura et al., 2008)). It is likely that Notch signalling, perhaps under synergistic regulation with the Wnt signalling pathway, controls the balance between LESC self-renewal and daughter cell commitment to differentiation. The cell cycle arrest transcription factor C/EBPΔ has also been implicated in the regulation of LESC self-renewal. Limbal epithelial basal cells that express C/EBPΔ co-express Bmi1 (which is involved in stem cell self renewal) and ΔNp63α (Barbaro et al., (Barbaro et al., 2007)).
Cell-cell communication is facilitated by gap junctions. Connexins 43 and connexin 50 are present in the corneal epithelium (Dong et al., (Dong et al., 1994)). Cx 43 is expressed by corneal basal cells except that of the limbus, implying it is utilised by more early TACs. The lack of intracellular communication has been suggested to help maintain stem cells and their niche (Matic et al., (Matic et al., 1997)) by protecting the cells from damage affecting adjacent neighbours (Chee et al., (Chee et al., 2006)). Like the stratified squamous epithelia, (Watt and Green, (1981)) involucrin is also expressed in the corneal epithelium (Chen et al., (Chen et al., 2004)) and in larger cells in vitro suggesting it is a marker of differentiation.
The RNA binding protein, Musashi-1 is produced in the developing and adult eye (Raji et al., (Raji et al., 2007)) and has recently been found in putative LESCs co-cultured with amniotic epithelial cells as feeders (Chen et al., (Chen et al., 2007)).
7.3. Clinical consequences of LESC failure and cultured stem cell therapy
LESC deficiency can occur as a result of primary or acquired insults. Partial or full LESC deficiency leads to deleterious effects on corneal wound healing and surface integrity (Chen and Tseng, (1991); Dua et al., (Dua et al., 2003)). Deficiency can arise following injuries including chemical or thermal burns and through diseases such as aniridia and Stevens Johnson syndrome (see Figure 5). As a result of LESC deficiency conjunctivalisation, neovascularisation, chronic inflammation, recurrent erosions, ulceration and stromal scarring can occur causing painful vision loss (Holland and Schwartz, (1996); Kenyon and Tseng, (1989); Puangsricharern and Tseng, (1995)). Long term restoration of visual function requires renewal of the corneal epithelium, through replacement of the stem cell population has traditionally been achieved by grafting limbal auto- or allografts (Kenyon and Tseng, (1989) {Ramaesh, 2003 #1138). Each procedure carries a risk of complication such as damage to healthy eye by removal of autologous tissue for transplantation or side effects from long-term immunosuppression with allogenic tissue. As an alternative, cultured LESC therapy has been developed where LESCs are expanded in vitro for therapeutic application in patients in a variety of protocols utilising amniotic membrane or fibrin, in the presence or absence of growth arrested 3T3 fibroblast feeder layers (Lindberg et al., (Lindberg et al., 1993); Pellegrini et al., (Pellegrini et al., 1997); Grueterich et al., (Grueterich et al., 2002); Koizumi et al., (Koizumi et al., 2001); Shortt et al., (Shortt, A.J. Secker, G.A. Notara, M.D. Limb, G.A. Khaw, P.T. Tuft, S.J. Daniels, J.T. (2007b). Transplantation of ex-vivo cultured limbal epithelial stem cells – a review of current techniques and clinical results. Surv Ophthalmol 52, 483–502. Article); Tsai et al., (Tsai et al., 2000)). Cultured autologous mucosal epithelial cell grafts have also been used to reconstruct the ocular surface of LESC deficient patients with some success (Nakamura et al., (Nakamura et al., 2003)). Recently it has been demonstrated that other stem cell populations including human embryonic stem cells (Ahmad et al., (Ahmad et al., 2007)) and hair follicle stem cells (Blazejekska et al., (Blazejekska et al., 2008)) can be driven towards a corneal epithelial-like phenotype. These exciting data may lead to alternative therapeutic strategies in the future for patients blinded by ocular surface disease cause by failure of LESC function.
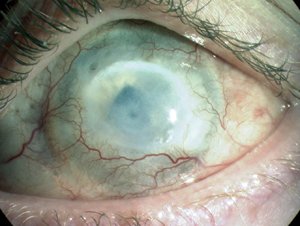
Figure 5. Chemical burn injury. An alkali burn to the human cornea can cause ocular surface failure with neovascularisation, opacification and blindness resulting from LESC deficiency.
The biological mechanisms of efficacy experienced by recipients of the cultured LESCs are unclear yet the clinical results are promising. It has been suggested that bone marrow derived stem cells may be recruited to the cornea to repair the damage caused by LESC failure (Daya et al., (Daya et al., 2005)) since no long-term survival of allogeneic cultured LESCs has been demonstrated. Our hypothesis is that the transplanted cultured limbal epithelium may act, at least in some patients, by ‘kick-starting’ the recipient's own ailing LESC.
One of the causes of blindness in children with aniridia is due to progressive ocular surface failure. The majority of cases are caused by PAX6 haploinsufficiency being a result of heterozygous null mutations (Van Heyningen and Williamson, (2002)). The disease is a pan-ocular, bilateral condition most prominently characterised by iris hypoplasia and varies from a relatively normal iris to the complete lack of an iris. Aniridia is often associated with cataracts, corneal vascularisation and glaucoma, with a significant number of cases of visual morbidity being due to corneal abnormalities. The underlying process of these abnormalities is poorly understood and is thought to be due to stem cell failure (Mackman et al., (Mackman et al., 1979); Nishida et al., (Nishida et al., 1995); Tseng and Li, (1996)). However, it has also been proposed that it may be due to a deficiency in the stem cell niche and adjacent corneal stroma (Ramaesh et al., (Ramaesh et al., 2005)). More recently downregulation of Pax6 has been linked to abnormal epidermal differentiation of cornea epithelial cells (Li et al., (Li et al., 2008)). Treatment usually involves replacement of LESC using limbal allografts and/or corneal grafts or more recently ex vivo cultured LESC grafts (Holland et al., (Holland et al., 2003)).
Aniridia represents a spectrum of disease, with iris anatomy defects ranging from the total absence of the iris to mild stomal hypoplasia with a pupil of normal appearance. Other associated defects include foveal hypoplasia, optic nerve hypoplasia, nystagmus, glaucoma and cataracts. These conditions may develop with age causing progressive visual loss. Another important factor leading to progressive loss of vision is aniridic-related keratopathy (ARK; Mackman et al., (Mackman et al., 1979); Margo, (1983)) which occurs in 90% of patients. Initially the cornea of patients appears normal during childhood (Nelson et al., (Nelson et al., 1984); Nishida et al., (Nishida et al., 1995)). Changes occur in patients in their early teenage years, with the disease manifesting as a thickened irregular peripheral epithelium. This is followed by superficial neovascularisation and if left untreated it may result in subepithelial fibrosis and stromal scarring. Furthermore patients develop recurrent erosions, ulcerations, chronic pain and eventual blindness (Holland et al., (Holland et al., 2003)). Histologically, stromal neovascularisation and infiltration of inflammatory cells is seen with the destruction of Bowman's layer. Additionally, the presence of goblet and conjunctival cells is seen on the corneal surface (Margo, (1983)). Traditionally, these clinical and histological manifestations have lead to the consensus that LESC deficiency is largely responsible for corneal abnormalities in aniridia (Dua and Azuara-Blanco, (2000); Margo, (1983)).
Based on the clinical and histological manifestation of aniridia, LESC deficiency has been presumed to be the pathogenesis behind ARK (Dua et al., (Dua et al., 2000); Margo, (1983); Nishida et al., (Nishida et al., 1995)). As a LESC marker has yet to be definitively identified, a true demonstration of LESC deficiency can not be assumed. Furthermore treatment for these patients involving replacement of LESC, either by keratolimbal allografts or more recently ex vivo expanded LESC grafts, provides a better outcome than corneal transplants (Holland et al., (Holland et al., 2003); Shortt et al., (Shortt, A.J. Secker, G.A. Notara, M.D. Limb, G.A. Khaw, P.T. Tuft, S.J. Daniels, J.T. (2007b). Transplantation of ex-vivo cultured limbal epithelial stem cells – a review of current techniques and clinical results. Surv Ophthalmol 52, 483–502. Article); Tiller et al., (Tiller et al., 2003)). This is consistent with LESC deficiency. However, patients who receive both limbal and corneal tissue seem to have the better outcome, suggesting an abnormality with corneal tissue and not just the limbus. This may be a downstream effect of LESC deficiency. Alternatively low levels of PAX6 may have a generalised effect on the entire cornea. ARK could also be the consequence of abnormal corneal epithelial/stromal healing responses as there is insufficient evidence to indicate that the proliferative potential of LESC is impaired (Ramaesh et al., (Ramaesh et al., 2005); Sivak et al., (Sivak et al., 2000)). Recently, studies looking at the regulation of genes downstream of Pax6 in the Pax6 heterozygous mouse, suggests the pathogenesis of ARK is due a number of mechanisms and not solely due to LESC deficiency (Ramaesh et al., (Ramaesh et al., 2005)). Further studies are needed to elucidate the exact mechanism of ARK progression to allow the use of appropriate treatments.
Mutations in Pax6 result in a distinct small eye syndrome in the small eye (SEY) mouse and rat (Hill et al., (Hill et al., 1991); Matsuo et al., (Matsuo et al., 1993)). These animals are excellent models for aniridia and the progressive nature of associated corneal abnormalities (Ramaesh et al., (Ramaesh et al., 2003); Davis et al., (Davis et al., 2003)). As the name suggests, mice with semidominant mutations develop small eyes and other ocular deformities. The murine strains Pax6Sey, Pax6SeyNeu and Pax6Coop represent three SEY mice with differing point mutations in the Pax6 gene. Pax6SeyDey and Pax6SeyH mice have Pax6 gene deletions (Hill et al., (Hill et al., 1991); Hogan et al., (Hogan et al., 1986); Lyon et al., (Lyon et al., 2000); Schmahl et al., (Schmahl et al., 1993); Theiler et al., (Theiler et al., 1980)). The SEY mice with semidominant heterozygous phenotypes demonstrate comparable developmental ocular abnormalities. This includes microophthalmia and defects in the iris, lens and retina with phenotypic severity being variable (Callearts et al., (Callearts et al., 1997); Hill et al., (Hill et al., 1991)). Cataracts, glaucoma and more importantly corneal abnormalities can develop in mutant SEY during post-natal development and adult life (Lyon et al., (Lyon et al., 2000)). Interestingly, the phenotypic variability seen between mice is also observed within a single SEY strain (Hogan et al., (Hogan et al., 1986)). This can even be detected between two eyes of the same mouse, suggesting a stringent requirement for Pax6 activity to be at specific levels at precise times during development (Hill et al., (Hill et al., 1991); Schedl et al., (Schedl et al., 1996); van Ramsdonk and Tilghman, (2000)). Homozygotes generate an ultimately lethal phenotype with no eyes and nasal primordial (Hill et al., (Hill et al., 1991)). A number of sey mice arose independently all of which are semidominant and by examining comparative mapping studies and phenotypic similarities to aniridia, it was suggested to be the mouse homologue of the human disease (Glaser T et al., 1990). This research led to the discovery that the Pax6 gene was responsible for the Sey phenotype and suggested that it was also responsible for the human disease, aniridia (Hill et al., (Hill et al., 1991)). These models are helping us to address fundamental questions about LESCs and their niche environment.
8. Summary
LESCs are clearly important for vision. Efforts to specifically and prospectively identify these elusive cells are proving difficult. However, despite this mixed populations of epithelial cells isolated from the limbal region have the potential to restore the ocular surface and improve vision in patients with LESC function failure. The mode of clinical efficacy (and treatment failure) may become apparent once a more thorough understanding of normal LESC regulation and the role of the niche is gained.
Copyright: © 2009 Genevieve A. Secker and Julie T. Daniels
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
§ To whom correspondence should be addressed. E-mail: j.daniels@ucl.ac.uk
* Edited by Fiona Watt and Fred Gage. Last revised May 28, 2009. Published June 30, 2009. This chapter should be cited as: Secker, G.A., and Daniels, J.T., Limbal epithelial stem cells of the cornea (June 30, 2009), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.48.1, [[UNSUPPORTED:p/uri]] .
References
- Ahmad, S. Stewart, R. Yung, S. Kolli, S. Armstrong, L. Stojkovic, M. Figueiredo, F. Lako, M. (2007). Differentiation of human embryonic stem cells into corneal epithelial like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells 25, 1145–1155. Abstract DOI
- Ambati, B. K. Nozaki, M. Singh, N. Takeda, A. Jani, P.D. Suthar, T. Alburquerque, R.J. Richter, E. Sakurai, E. Newcomb, M.T. et al. (2006). Corneal avascularity is due to soluble VEGF receptor-1. Nature 443, 993–997. Abstract DOI
- Ambati, B.K. Patterson, E. Jani, P. Jenkins, C. Higgins, E. Singh, N. Suthar, T. Vira, N. Smith, K. Caldwell, R. (2007). Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br J Ophthalmol 91, 505–508. Abstract DOI
- Arpitha, P. Prajna, N.V. Srinivasan, M. Muthukkaruppan, V. (2005). High expression of p63 combined with a large N/C ratio defines a subset of human limbal epithelial cells: implications on epithelial stem cells. Invest Ophthalmol Vis Sci 46, 3631–3636. Abstract DOI
- Barbaro, V. Testa, A. di Iorio, E. Mavilio, F. Pellegrini, G. de Luca, M. (2007). C/EBP delta regulates cell cycle and self-renewal of human limbal stem cells. J Cell Biol 177, 1037–1049. Abstract DOI
- Barrandon, Y. Green, H. (1987). Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA 84, 2302–2306. Abstract DOI
- Baum, J.L. (1970). Melanocyte and Langerhans cell population of the cornea and limbus in the albino animal. Am J Ophthalmol 69, 669–676. Abstract Abstract
- Beebe, D.C. Masters, B.R. (1996). Cell lineage and the differentiation of corneal epithelial cells. Invest Ophthalmol Vis Sci 37, 1815–1825. Abstract Abstract
- Bickenbach, J.R. (1981). Identification and behaviour of label-retaining cells in oral mucosa and skin. J Dent Res 60, 1611–1620. Abstract Abstract
- Blazejekska, E.A. Schlotzer-Schrehardt, U. Zenkel, M. Bachmann, B. Chankiewitz, E. Jacobi, C. Kruse, F.E. (2008). Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal-like epithelial cells. Stem Cells.
- Callearts, P. Halder, G. Gehring, W.J. (1997). PAX-6 in development and evolution. Annu, Rev Neurosci 20, 583–532.
- Calvi, L.M. Adams, G.B. Weibrecht, K.W. Weber, J.M. Olson, D.P. Knight, M.C. Martin, R.P. Schipani, E. Divieti, P. Bringhurst, F.R. et al. (2003). Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846. Abstract DOI
- Chaloin-Dufau, C. Sun, T.T. Dhouailly, D. (1990). Appearance of the keratin pair K3/K12 during embryonic and adult corneal epithelial differentiation in the chick and in the rabbit. Cell Differ Dev 32, 97–108. Abstract DOI
- Chee, K.Y. Kicic, A. Wiffen, S.J. (2006). Limbal stem cells: the search for a marker. Clin Exp Ophthalmol 34, 64–73. Article DOI
- Chen, J.J. Tseng, S.C. (1991). Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci 32, 2219–2233. Abstract Abstract
- Chen, Z. de Paiva, C.S. Luo, L. Kretzer, F.L. Pflugfelder, S.C. Li, D.Q. (2004). Characterisation of putative stem cell phenotype in human limbal epithelia. Stem Cells 22, 355–366. Abstract DOI
- Chen, Y.T. Li, W. Hayashida, Y. He, H. Chen, S.Y. Tseng, D.Y. Kheirkhah, A. Tseng, S.C. (2007). Human amniotic membrane epithelial cells as novel feeder layers for promoting ex vivo expansion of epithelial progenitor cells. Stem Cells 25, 1995–2005. Abstract DOI
- Chung, E.H. Bukusoglu, G. Zieske, J.D. (1992). Localisation of corneal epithelial stem cells in the developing rat. Invest Ophthalmol Vis Sci 33, 2199–2206. Abstract Abstract
- Cintron, C. Covington, H. Kublin, C.L. (1983). Morphogenesis of rabbit corneal stroma. Invest Ophthalmol Vis Sci 24, 543–556. Abstract Abstract
- Clayton, E. Doupe, D.P. Klein, A.M. Winton, D.J. Simons, B.D. Jones, P.H. (2007). A single type of progenitor cell maintains normal epidermis. Natrure 446, 185–189. Article DOI
- Cotsarelis, G. Cheng, G. Dong, G. Sun, T.T. Lavker, R.M. (1989). Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209. Abstract DOI
- Coulombre, A. Coulombre, J. (1964). Lens development. The role of the lens in eye growth. J Exp Zo0 156, 39–48. Article DOI
- Davanger, M. Evenson, A. (1971). Role of the pericorneal structure in renewal of corneal epithelium. Nature 229, 560–561. Abstract DOI
- Davis, J. Duncan, M.K. Robison, W.G.J. Piatigorsky, J. (2003). Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci 116, 2157–2167. Abstract DOI
- Daya, S.M. Watson, A. Sharpe, J.R. Giledi, O. Rowe, A. James, S.E. (2005). Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology 112, 470–477. Abstract DOI
- Di Iorio, E. Barbaro, V. Ruzza, A. Ponzin, D. Pellegrini, G. de Luca, M. (2005). Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA 102, 9523–9528. Abstract DOI
- Dong, Y. Roos, M. Gruijters, T. Donaldson, P. Bullivant, S. Beyer, E. Kistler, J. (1994). Differential expression of two gap junctions proteins in corneal epithelium. Eur J Cell Biol 64, 95–100. Abstract Abstract
- Du, Y. Funderburgh, M. Mann, M.N. SunderRaj, N. Funderburgh, J.L. (2005). Multipotent stem cells in human corneal stroma. Stem Cells 23, 1266–1275. Abstract DOI
- Dua, H.S. Azuara-Blanco, A. (2000). Limbal stem cells of the corneal epithelium. Surv Ophthalmol 44, 415–425. Abstract DOI
- Dua, H.S. Saini, J.S. Azuara-Blanco, A. Gupta, P. (2000). Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol 48 Abstract Abstract
- Dua, H.S. Joseph, A. Shanmuganathan, V.A. Jones, R.E. (2003). Stem cell differentiation and the effects of deficiency. 17, 877–885.
- Ebato, B. Friend, J. Thoft, R.A. (1987). Comparison of central and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci 28, 1450–1456. Abstract Abstract
- Ebato, B. Friend, J. Thoft, R.A. (1988). Comparison of limbal and peripheral human corneal epithelium in tissue culture. Invest Ophthalmol Vis Sci 29, 1533–1537. Abstract Abstract
- Fini, M.E. Stramer, B.M. (2005). How the cornea heals: cornea-specific repair mechanism affecting surgical outcomes. Cornea 24, S2–S11. Abstract DOI
- Funderburgh, M.L. Du, Y. Mann, M.N. SunderRaj, N. Funderburgh, J.L. (2005). PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J 19, 1371–1373. Abstract Abstract
- Gipson, I.K. (1989). The epithelial basement membrane zone of the limbus. Eye 3, 132–140. Abstract Abstract
- Grueterich, M. Espana, E.M. Touhami, A. Ti, S.E. Tseng, S.C. (2002). Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology 109, 1547–1552. Abstract DOI
- Haustein, J. (1983). On the ultrastructure of the developing and adult mouse corneal stroma. Anat Embryol 168, 291–305. Abstract DOI
- Hay, E.D. Linsenmayer, T.F. Trelstad, R.L. von der Mark, K. (1979). Origin and distribution of collagens in the developing avian cornea. Curr Top Eye Res 1, 1–35. Abstract Abstract
- Hayashi, R. Yamato, M. Sugiyama, H. Sumide, T. Yang, J. Okano, T. Tano, Y. Nishida, K. (2007). N-cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells 25, 289–296. Abstract DOI
- Higa, K. Shimmura, S. Miyashita, H. Shimazaki, J. Tsubota, K. (2005). Melanocytes in the corneal limbus intercat wirh K19-positive epithelial cells. Exp Eye Res 81, 218–223. Abstract DOI
- Hill, R.E. Favor, J. Hogan, B.L. Ton, C.C. Saunders, G.F. Hanson, I.M. Prosser, J. Jordan, T. Hastie, N.D. van Heyningen, V. (1991). Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, 522–525. Abstract DOI
- Hogan, B.L. Horsburgh, G. Cohen, J. Hetherington, C.M. Fisher, G. Lyon, M.F. (1986). Small eyes (Sey): a homozygous lethal mutation on chromosaome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol 97, 95–110. Abstract Abstract
- Holland, E.J. Schwartz, G.S. (1996). The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea 15, 549–556. Abstract DOI
- Holland, E.J. Djalilian, A.R. Schwartz, G.S. (2003). Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology 110, 125–130. Abstract DOI
- Huang, A.J. Tseng, S.C. (1991). Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci 32, 96–105. Abstract Abstract
- Jester, J.V. Moller-Pedersen, T. Huang, J. Sax, C.M. Kays, W.T. Cavanagh, H.D. Petroll, W.M. Piatigorsky, J. (1999). The cellular basis of corneal transparency: evidence for corneal crystrallins. J Cell Sci 112, 613–622. Abstract Abstract
- Jones, P.H. Watt, F.M. (1993). Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724. Abstract DOI
- Jones, P.H. Simon, B.D. Watt, F.M. (2007). Sic transit gloria: farewell to the epidermal transit amplifying cell?. Cell Stem Cell 1, 371–381. Abstract DOI
- Kaufman, M.H. (1992). Postcranial morphological features of homozygous tetraploid mouse embryos. J Anat 180, 521–534. Abstract Abstract
- Kenyon, K.R. Tseng, S.C. (1989). Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96, 709–722. Abstract Abstract
- Kinoshita, S. Friend, J. Thoft, R.A. (1981). Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci 21, 434–441. Abstract Abstract
- Klenkler, B. Sheardown, H. (2004). Growth factors in the anterior segment: role in tissue maintenance, wound healing and ocular pathology. Exp Eye Res 79, 677–688. Abstract DOI
- Klyce, S.D. (1972). Electrical profiles in the corneal epithlelium. J Physiol 226, 407–429. Abstract Abstract
- Koizumi, N. Inatomi, T. Suzuki, K. Sotozono, C. Kinoshita, S. (2001). Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 108, 1569–1574. Abstract DOI
- Kruse, F.E. Tseng, S.C. (1993). A tumor promoter-resistant subpopulation of progenitor cells is larger in limbal epitheliumm than in corneal epithelium. Invest Ophthalmol Vis Sci 34, 2501–2511. Abstract Abstract
- Kurpakus, M.A. Stock, E.L. Jones, J.C. (1990). Expression of the 55-kD/64-kD corneal keratins in ocular surface epithelium. Invest Ophthalmol Vis Sci 31, 448–456. Abstract Abstract
- Lavker, R.M. Dong, G. Cheng, S.Z. Kudoh, K. Cotsarelis, G. Sun, T.T. (1991). Relative proliferative rates of limbal and corneal epithelia. Implications of corneal epithelial migration, circadian rhythm, and suprabasally located DNA-sunthesising keratinocytes. Invest Ophthalmol Vis Sci 32, 1864–1875. Abstract Abstract
- Lavker, R.M. Wei, Z.G. Sun, T.T. (1998). Phorbol ester preferentially stimulates mouse forniceal conjunctival and limbal epithelial cells to proliferate in vivo. Invest Ophthalmol Vis Sci 39, 301–307. Abstract Abstract
- Lavker, R.M. Sun, T.T. (2003). Epithelial stem cells: the eye provides a vision. Eye 17, 937–942. Abstract DOI
- Lawrenson, J.G. Ruskell, G.L. (1991). The structure of corpuscular nerve endings in the limbal conjunctiva of the human eye. J Anat 177, 75–84. Abstract Abstract
- Lehrer, M.S. Sun, T.T. Lavker, R.M. (1998). Strategies of epithelial repair: modulation o stem cell and transit amplifying cell proliferation. J Cell Sci 111, 2867–2875. Abstract Abstract
- Li, T. Lu, L. (2005). Epidermal growth factor-induced proliferation requires down-regulation of Pax6 in corneal epithelial cells. J Biol Chem 280, 12988–12995. Abstract DOI
- Li, W. Chen, Y.-T. Hayashida, Y. Blanco, G. Kheirkah, A. He, H. Chen, S.-Y. Liu, C.Y. Tseng, S.C. (2008). Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial surface diseases. J Pathol 214, 114–122. Abstract DOI
- Lindberg, K. Brown, M.E. Chaves, H.V. Kenyon, K.R. Rheinwald, J.G. (1993). In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci 34, 2672–2679. Abstract Abstract
- Ljubimov, A.V. Burgeson, R.E. Butkowski, R.J. Micheal, A.F. Sun, T.T. Kenney, M.C. (1995). Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 72, 461–473. Abstract Abstract
- Lyon, M.F. Bogani, D. Boyd, Y. Guillot, P. Favor, j. (2000). Further genetic analysis of two autosomal dominant mouse eye defects, Ccw and Pax6(coop). Mol Vis 6, 199–203. Abstract Abstract
- Ma, A. Boulton, M. Zhao, B. Connon, C. Cai, J. Albon, J. (2007). A role for notch signalling in human corneal epithelial cell differentiation and proliferation. Invest Ophthalmol Vis Sci 48, 3576–3585. Abstract DOI
- Mackman, G. Brightbill, F.S. Optiz, J.M. (1979). Corneal changes in aniridia. Am J Ophthalmol 87, 497–502. Abstract Abstract
- Majo, F. Rochat, A. Nicolas, M. Jacoude, G.A. Barrandon, Y. (2008). Oligopotent stem cells are distributed througout the entire mammalian ocualr surface. Nature 456, 250–254. Abstract DOI
- Mann, I. (1944). A study of epithelial regenration in the living eye. Br J Ophthalmol 28, 26–40. Abstract DOI
- Margo, C.E. (1983). Congenital aniridia: a histopathologic study of the anterior segment in children. J Pediatr Ophthalmol Strabismus 20, 192–198. Abstract Abstract
- Matic, M. Petrov, I.N. Chen, S. Wang, C. Dimitrijevich, S.D. Wolosin, J.M. (1997). Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation 61, 251–260. Abstract DOI
- Matsuo, T. Osumi-Yamashita, N. Noji, S. Ohuchi, H. Koyama, E. Myokai, F. Matsuo, N. Taniguchi, S. Doi, H. Iseke, S. (1993). A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nat Genet 3, 299–304. Abstract DOI
- Meek, K.M. Dennis, S. Khan, S. (2003). Changes in the refractive index of the stroma and its extracellular matrix when the cornea swells. Biophys J 85, 2205–2212. Abstract DOI
- Miyashita, H. Higa, K. Kato, N. Kawakita, T. Yoshida, S. Tsubota, K. Shimmura, S. (2007). Hypoxia enhances the expansion of human limbal epithelial progenitor cells in vitro. Invest Ophthalmol Vis Sci 8
- Mukhopadhyay, M. Gorivodsky, M. Shtrom, S. Grinberg, A. Niehrs, C. Morasso, M.I. Westpal, H. (2006). Dkk2 playa an essential role in the corneal fate of the ocualr surface epithelium. Development 133, 2149–2154. Abstract DOI
- Muller, L.J. Pels, L. Vrensen, G.F. (1995). Novel aspects of the ultrastructural organisation of human corneal keratocytes. Invest Ophthalmol Vis Sci 36, 2557–2567. Abstract Abstract
- Nakamura, M. Endo, K.-I. Cooper, L.J. Fullwood, N. Tanifuji, N. Tsuzuki, M. Koziumi, N. Inatomi, T. Sano, Y. Kinoshita, S. (2003). The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 44, 106–116. Abstract DOI
- Nakamura, M. Ishikawa, F. Sonoda, K.H. Hisatomi, T. Qiao, H. Yamada, J. Fukata, M. Ishibashi, T. Harada, M. Kinoshita, S. (2005). Characterisation and distribution of bone marrow stem cells in the mouse cornea. Invest Ophthalmol Vis Sci 46, 497–503. Abstract DOI
- Nakamura, T. Ohtsuka, T. Sekiyama, E. Cooper, L.J. Kokubu, H. Fullwood, N. Barrandon, Y. Kageyama, R. Kinoshita, S. (2008). Hes1 regualtes corneal developmentand the function of corneal stem/progenitor cells. Stem Cells 26, 1265–1274. Abstract DOI
- Nelson, L.B. Spaeth, G.L. Nowinski, T.S. Margo, C.E. Jackson, L. (1984). Aniridia. A review. Surv Ophthalmol 28, 621–642. Abstract DOI
- Nishida, K. Kinoshita, S. Ohashi, Y. Kuwayama, Y. Yamamoto, S. (1995). Ocular surface abnormalities in aniridia. Am J Ophthalmol 120, 368–375. Abstract Abstract
- Pajoohesh-Ganji, A. Stepp, M.A. (2005). In search of markers for the corneal epithelium. Biol Cell 97, 265–276. Abstract DOI
- Pei, Y.F. Rhodin, J.A. (1970). The prenatal development of the mouse eye. Anat Rec 168, 105–125. Abstract DOI
- Pellegrini, G. Traverso, C.E. Franzi, A.T. Zingirian, M. Cancedda, R. De Luca, M. (1997). Long-term restoration of damaged corneal surfaces with autologous cultivated human epithelium. The Lancet 349, 990–993. Article DOI
- Pellegrini, G. Golisano, O. Paterna, P. Lambiase, A. Bonini, S. Rama, P. De Luca, M. (1999). Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 145, 769–782. Abstract DOI
- Pellegrini, G. Dellambra, E. Golisano, O. Martinelli, E. Fantozzi, I. Bondanza, S. Ponzin, D. McKeon, F. De Luca, M. (2001). p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA 98, 3156–3161. Abstract DOI
- Puangsricharern, V. Tseng, S.C. (1995). Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology 102, 1476–1485. Abstract Abstract
- Qi, H. Li, D.Q. Shine, H.D. Chen, Z. Yoon, K.C. Jones, D.B. Pflugfelder, S.C. (2008). Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res 86, 34–40. Abstract DOI
- Raji, B. Dansault, A. Leemput, J. de la Houssaye, G. Vieira, V. Kobetz, A. Arbogast, L. Masson, C. Menasche, M. Abitbol, M. (2007). The RNA-binding protein Musashi-1 is produced in the developing and adult mouse eye. Mol Vis 10, 1412–1427.
- Ramaesh, T. Collinson, J.M. Ramaesh, K. Kaufman, M.H. West, J.D. Dhillon, B. (2003). Corneal abnormalities in Pax6 +/- small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci 44, 1871–1878. Abstract DOI
- Ramaesh, K. Ramaesh, T. Dutton, G.N. Dhillon, B. (2005). Evolving concepts on the pathogenic mechanisms of aniridia related keratopathy. Int J Biochem Cell Biol 37, 547–557. Abstract DOI
- Reneker, L.W. Silversides, D.W. Patel, K. Overbeek, P.A. (1995). TGF alpha can act as a chemoattractant to perioptic mesenchymal cells in developing mouse eyes. Development 121, 1669–1680. Abstract Abstract
- Reneker, L.W. Silversides, D.W. Xu, L. Overbeek, P.A. (2000). Formation of corneal endothelium is essential for anterior segment development – a transgenic mouse model of anterior segment. Development 127, 533–542. Abstract Abstract
- Robinson, M.L. Ohtaka-Maruyama, C. Chan, C.C. Jamieson, S. Dickson, C. Overbeek, P.A. Chepelinsky, A.B. (1998). Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol 198, 13–31. Abstract DOI
- Rolando, M. Zierhut, M. (2001). The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 45, S203–S210. Abstract DOI
- Romano, A.C. Espana, E.M. Yoo, S.H. Budak, M.T. Wolosin, J.M. Tseng, S.C. (2003). Different cell sized in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci 44, 5125–5129. Abstract DOI
- Schedl, A. Ross, A. Lee, M. Engelkamp, D. Rashbass, P. Van Heyningen, V. Hastie, N.D. (1996). Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell 86, 71–82. Abstract DOI
- Schermer, A. Galvin, S. Sun, T.T. (1986). Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103, 49–62. Abstract DOI
- Schlotzer-Schrehardt, U. Kruse, F.E. (2005). Identification and characterisation of limbal stem cells. Exp Eye Res 81, 247–264. Abstract Abstract
- Schlotzer-Schrehardt, U. Dietrich, T. Saito, K. Sorokin, L. Sasaki, T. Paulsson, M. Kruse, F.E. (2007). Characterisation of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res 85, 845–860. Abstract DOI
- Schmahl, W. Knoedlseder, M. Favor, J. Davidson, D. (1993). Defects of neuronal migration and the pathogenesis of corticol malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6 locus. Acta Neuropathol 86, 126–135. Abstract DOI
- Schofield, R. (1983). The stem cell system. Biomed Pharmacother 37, 375–380. Abstract Abstract
- Sharma, A. Coles, W.H. (1989). Kinetics of corneal epithelial maintenance and graft loss. A population balance model. Invest Ophthalmol Vis Sci 30, 1962–1971. Abstract Abstract
- Shimmura, S. Tsubota, K. (1997). Ultraviolet B-induced mitochondrial dysfunction is associated with decreased cell detachment of corneal epithelial cells in vitro. Invest Ophthalmol Vis Sci 38, 620–626. Abstract Abstract
- Shimmura, S. Miyashita, H. Higa, K. Yoshida, S. Shimazaki, J. Tsubota, K. (2006). Proteoic analysis of soluble factors secreted by limbal fibroblasts. Mol Vis 11, 478–484.
- Shortt, A.J. Secker, G.A. Munro, P.M. Khaw, P.T. Tuft, S.J. Daniels, J.T. (2007a). Characterisation of the limbal epithelial stem cell niche: novel imaging techniques permit in-vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 5, 1402–1409. Article DOI
- Shortt, A.J. Secker, G.A. Notara, M.D. Limb, G.A. Khaw, P.T. Tuft, S.J. Daniels, J.T. (2007b). Transplantation of ex-vivo cultured limbal epithelial stem cells – a review of current techniques and clinical results. Surv Ophthalmol 52, 483–502. Article DOI
- Sivak, J.M. Mohan, R. Rinehart, W.B. Xu, P.X. Maas, R.L. Fini, M.E. (2000). Pax-6 expression and activity are induced in the reepithelialising cornea and control activity of the transcriptional promotor for matrix metalloproteinase gelatinase B. Dev Biol 222, 41–54. Abstract DOI
- Song, J. Lee, Y.G. Houston, J. Petroll, W.M. Chakravarti, S. Cavanagh, H.D. Jester, J.V. (2003). Neonatal corneal stromal development in the normal and lumican deficient mouse. Invest Ophthalmol Vis Sci 44, 548–457. Abstract DOI
- Stepp, M.A. Zhu, L. Sheppard, D. Cranfill, R.L. (1995). Localised distribution of alpha 9 integrin in the cornea and changes in expression during corneal epithelial cell differentiation. J Histochem Cytochem 43, 353–362. Abstract Abstract
- Stepp, M.A. Zhu, L. (1997). Upregulation of alpha 9 integrin and tenascin during epithelial regeneration after debridement in the cornea. J Histochem Cytochem 45, 189–201. Abstract Abstract
- Sun, L. Ryan, D.G. Zhou, M. Sun, T.T. Lavker, R.M. (2006). EEDA: a protein associated with an early stage of stratified epithelial differentiation. J Cell Physiol 206, 103–111. Abstract DOI
- Theiler, K. Varnum, D.S. Stevens, L.C. (1980). Development of Dickie's small eye: an early lethal mutation in the house mouse. Anat Embryol 161, 115–120. Abstract DOI
- Thoft, R.A. Friend, J. (1983). The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 24, 1442–1443. Abstract Abstract
- Thomas, P.B. Liu, Y.H. Zhuang, F.F. Selvam, S. Song, S.W. Smith, R.E. Trousdale, M.D. Yiu, S.C. (2007). Identification of Notch-1 expression in the limbal basal epithelium. Mol Vis 13, 337–344. Abstract Abstract
- Tiller, A.M. Odenthal, M.T. Verbraak, F.D. Gortzak-Moorstein, N. (2003). The influence of keratoplasty on visual prognosis in aniridia: a historical review of one large family. Cornea 22, 105–110. Abstract DOI
- Trainor, P.A. Tam, P.P. (1995). Cranial papaxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial mesenchyme but distinct segregation in brachial arches. Development 121, 2569–2582. Abstract Abstract
- Tsai, R.J.-F. Li, L.-M. Chen, J.-K. (2000). Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. New Engl J Med 343, 86–93. Abstract DOI
- Tseng, S.C. (1989). Concept and application of limbal stem cells. Eye 3, 141–157. Abstract Abstract
- Tseng, S.C. Li, D.Q. (1996). Comparison of protein kinase C subtype expression between normal and aniridic human ocular surfaces: implications for limbal cell dysfunction in aniridia. Cornea 15, 168–178. Abstract DOI
- Van Heyningen, V. Williamson, K.A. (2002). PAX6 in sensory development. Hum Mol Genet 11, 1161–1167. Abstract DOI
- Vantrappen, L. Geboes, K. Missotten, L. Maudgal, P.C. Desmet, V. (1985). Lymphocytes and Langerhans cells in the normal cornea. Invest Ophthalmol Vis Sci 26, 220–225. Abstract Abstract
- Vauclair, S. Majo, F. Durham, A.D. Ghyselinck, N.B. Barrandon, Y. Radke, F. (2007). Corneal epithelial cell fate is maintained during repair by Notch-1 signalling via the regulation of vitamin A metabolism. Dev Cell 13, 242–253. Abstract DOI
- Watanabe, H. Tisdale, A.S. Gipson, I.K. (1993). Eyelid opening induces expression of a glycocalyx glycoprotein of rat ocular surface epithelium. Invest Ophthalmol Vis Sci 34, 327–338. Abstract Abstract
- Watanabe, K. Nishida, K. Yamato, M. Umemoto, T. Sumide, T. Yamamoto, K. Maeda, N. Watanabe, H. Okano, T. Tano, Y. (2004). Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett 565, 6–10. Abstract DOI
- Watt, F.M. Green, H. (1981). Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol 90, 738–742. Abstract DOI
- Watt, F.M. Hogan, B.L. (2000). Out of eden: stem cells and their niches. Science 287, 1427–1430. Abstract DOI
- Welge-Lussen, U. May, C.A. Neubauer, A.S. Priglinger, S. (2001). Role of tissue growth factors in aqueous humor homestasis. Curr Opin Ophthalmol 12, 94–99. Abstract DOI
- West-Mays, J.A. Dwivedi, D.J. (2006). The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol 38, 1625–1631. Abstract DOI
- Zhang, J. Niu, C. Ye, L. Huang, H. He, X. Tong, W.G. Ross, J. Haug, J. Johnson, T. Feng, J.Q. et al. (2003). Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841. Abstract DOI
- Zhou, S. Schuetz, J.D. Bunting, K.D. Colapietro, A.M. Sampath, J. Morris, J.J. Lagutina, I. Grosveld, G.C. Osawa, M. Nakauchi, H. Sorrentino, B.P. (2001). The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of a side-population phenotype. Nat Med 7, 1028–1034. Abstract DOI
- Zieske, J.D. Guimaraes, S.R. Hutcheon, A.E. (2001). Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res 72, 33–39. Abstract DOI
- de Paiva, C.S. Chen, Z. Corrales, R.M. Pflugfelder, S.C. Li, D.Q. (2005). ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 23, 63–73. Abstract DOI
- van Ramsdonk, C.D. Tilghman, S.M. (2000). Dosage requiremenr and allelic expression of PAX6 during lens placode formation. Development 127, 5439–5448. Abstract Abstract

