C. elegans germline stem cells are a particularly simple system for analysis of stem cell regulation. Their well-defined mesenchymal niche consists of a single cell, the Distal Tip Cell, which uses Notch signaling to maintain a pool of germline stem cells. Downstream of Notch signaling a post-transcriptional regulatory network dictates self-renewal or differentiation. The major self-renewal hub of that network is FBF, a conserved RNA-binding protein and conserved stem cell regulator. FBF represses mRNAs encoding key regulators of germline differentiation (entry into the meiotic cell cycle, sperm or oocyte specification) as well as established regulators of somatic differentiation. Transcriptional and post-transcriptional mechanisms also control totipotency in the C. elegans germline. The key C. elegans GSC regulators are conserved broadly, making this system a paradigm for stem cell regulation.
1. Introduction
Germ cells have many common features. Their stem cells generate the germline tissue during development and often maintain it in adults; their cell fate decisions include entry into the meiotic cell cycle and specialization as sperm or egg; and they retain the potential for totipotency (gametes produce all cell types in the next generation). Because germ cells are an ancient cell type and share these many traits, their developmental regulation is likely based on an ancient program that has been modified during evolution to achieve phyla- and species-specific character.
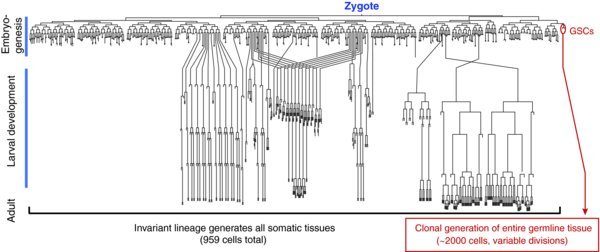
Figure 1. Complete C. elegans lineage. The C. elegans zygote embarks on an invariant cell lineage to generate all somatic tissue plus two germline stem cells (GSCs). GSCs subsequently divide variably to make the germline tissue during larval development and to maintain it in adults ((Kimble et al., 1979); (Sulston et al., 1977); (Sulston et al., 1983)).
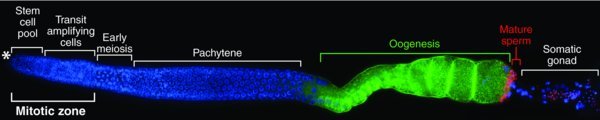
Figure 2. Adult germline organization. A single gonadal arm has been extruded from an adult hermaphrodite and stained with DAPI (blue) to visualize nuclei, an oocyte marker (green) and a sperm marker (red). An asterisk (*) marks the distal end, where the Distal Tip Cell forms the niche for GSCs (see Figure 3). Each hermaphrodite has two such arms, each with ∼1000 germ cells. Germ cells mature in a linear pattern from distal to proximal with GSCs most distal and gametes most proximal. The somatic gonadal cells in each gonadal arm include a single DTC (see Figure 3), two sheath cells that partially wrap germ cells in meiotic prophase, eight myoepithelial cells that surround the oogenic region, and the spermatheca and uterus more proximally. Only the spermatheca is easily visible in this image. Micrograph from Kyung Won Kim (Kimble lab).
This chapter describes the molecular regulation of germline self-renewal, cell fate decisions, and totipotency in the nematode Caenorhabditis elegans. Our understanding of C. elegans germline stem cell (GSC) regulation began in 1981 with discovery of their mesenchymal stem cell niche and progressed through the years with discoveries of molecular mechanisms of niche signaling and a downstream regulatory network with many counterparts in other stem cell systems. The key C. elegans GSC regulators are conserved broadly, making this system a paradigm for stem cell regulation. Although much is known about C. elegans GSC regulation, major questions remain.
2. C. elegans germline stem cells and their niche
C. elegans can exist as either a self-fertilizing XX hermaphrodite or a cross-fertilizing XO male. Most studies of GSCs have been conducted using hermaphrodites, and for simplicity, we focus on hermaphrodites in this chapter. Nonetheless, the principal mechanisms regulating GSCs are similar in the two sexes, though some details differ ((Kimble et al., 2007); (Kimble et al., 1979); (Kimble et al., 1981); (Morgan et al., 2010); (Sulston et al., 1983)).
2.1. C. elegans germline stem cells and germline organization
C. elegans GSCs generate the germline tissue during development ((Kimble et al., 1979)), maintain it in adults ((Crittenden et al., 2006)), and regenerate it after periods of starvation ((Angelo et al., 2009); (Seidel et al., 2011)). First-stage (L1) larvae are born with two GSCs, which generate the adult germline clonally (Fig. 1) ((Kimble et al., 1979)). Proliferation of the GSCs during larval development generates an adult germline containing ∼1,000 germ cells in each of the hermaphrodite's two gonadal arms. The adult germline is organized in a simple linear fashion with self-renewal at the distal end and differentiating gametes at the proximal end (Fig. 2). Lineage analysis of adult GSCs has not been technically possible, but multiple lines of evidence suggest existence of a stem cell pool with ∼35–70 GSCs at the distal end of the germline tissue (e.g. (Cinquin et al., 2010); (Crittenden et al., 2006)). Both larval and adult C. elegans GSCs divide variably with respect to orientation and asynchronously in time and do not use stereotyped asymmetric cell divisions ((Crittenden et al., 2006); (Kimble et al., 1979)).
2.2. The Distal Tip Cell forms a niche for GSCs
The niche for GSCs consists of a single mesenchymal cell, called the Distal Tip Cell (DTC) (Fig. 3). Each hermaphrodite has two DTCs, one located at the distal end of each gonadal arm (Fig. 2, asterisk; Fig. 3). The large cell body of the DTC encapsulates the tip of the gonad, and an elaborate network of DTC processes extends along the gonad proximally, ending at the boundary of early meiotic entry (Fig. 3).
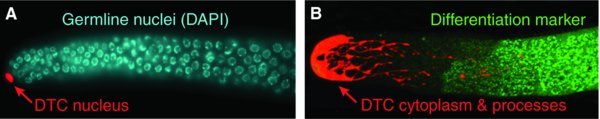
Figure 3. Single-celled Distal Tip Cell niche for GSCs. A. The DTC nucleus (red), highlighted with nuclear mCherry, is localized at the distal end. Germ cell nuclei visualized using DAPI. Micrograph from Hannah Seidel (Kimble lab). B. DTC (red), highlighted with cytoplasmic GFP, shows cell body at the distal end and DTC processes extending proximally. The onset of germline differentiation is observed using the GLD-1 marker (green). Micrograph from Karla Knobel (Kimble lab).
Remarkably, the DTC is responsible for maintaining GSCs throughout the life of the animal. Ablation of the DTC at any stage of development causes all GSCs to enter the meiotic cell cycle and differentiate ((Kimble et al., 1981)). Furthermore, although the orientation of GSC cell divisions is variable, the destiny of GSC progeny is reproducible relative to the niche: daughters adjacent to the DTC remain undifferentiated, whereas daughters born at a distance from the DTC ultimately enter the meiotic cell cycle and differentiate ((Crittenden et al., 2006); (Kimble et al., 1981); (McGovern et al., 2009)). Thus, the fate of GSC progeny is determined by their proximity to the DTC; through this spatially-defined mechanism, the DTC controls generation of the adult germline during larval development as well as replenishment of this tissue during adulthood.
3. Molecular regulation of GSC self-renewal
Key molecular regulators of GSCs have been identified by their dramatic effects on self-renewal: their removal causes GSCs to cease self-renewal and differentiate. These regulators have been analyzed genetically to understand regulatory relationships, biochemically to learn molecular mechanisms, and microscopically to place them in cellular context. As a result, GSC regulation is now reasonably well understood, although some intriguing gaps remain.
3.1. The DTC uses Notch signaling to maintain GSCs
The DTC niche maintains GSCs through GLP-1/Notch signaling. The canonical Notch signaling pathway employs similar core components in all animals: a DSL transmembrane ligand, a transmembrane Notch receptor, and a pathway-specific transcription factor complex to activate transcription (see (Kopan et al., 2009) for details). Figure 4 diagrams these components in the distal gonad of C. elegans: the DTC expresses the DSL ligands LAG-2 and APX-1, and GSCs express the Notch receptor GLP-1 ((Crittenden et al., 1994); (Henderson et al., 1994); (Nadarajan et al., 2009); (Tax et al., 1994)). After cleavage, the intracellular domain of the GLP-1/Notch receptor works with a CSL DNA-binding protein, LAG-1, and a transcriptional co-activator, LAG-3/SEL-8, to activate transcription ((Christensen et al., 1996); (Doyle et al., 2000); (Petcherski et al., 2000)).
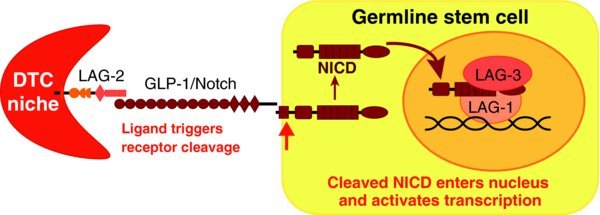
Figure 4. DTC niche uses Notch signaling to maintain GSCs. LAG-2 ligand from DTC niche activates proteolytic cleavage of GLP-1 receptor in germline stem cells. This cleavage generates the Notch intracellular domain (NICD), which enters the nucleus to form a ternary complex with LAG-1, a CSL DNA-binding protein, and LAG-3/SEL-8, a Mastermind-like transcriptional co-activator.
The importance of GLP-1/Notch signaling for GSC maintenance is demonstrated by the effects of either GLP-1/Notch removal or over-activity. Removal of the GLP-1/Notch receptor mimics DTC ablation: all GSCs enter the meiotic cell cycle and differentiate ((Austin et al., 1987)). This fate transformation occurs in early larvae in the glp-1 null mutant, but also is observed after removal of GLP-1 at any stage, which can be accomplished using staged temperature shifts of temperature-sensitive glp-1 mutants or staged RNAi. Conversely, in glp-1 gain-of-function mutants, where the GLP-1 receptor signals independently of the ligand, meiotic entry does not occur; instead, germlines become tumorous and fill with mitotically dividing cells ((Berry et al., 1997)). Thus, GLP-1/Notch signaling is required continuously for GSC maintenance throughout post-embryonic development, and the typical confinement of GSCs to the distal end of the gonad relies on localized restriction of DSL ligands to that end.
In addition to the classic case of C. elegans GSCs, Notch signaling controls stem cells in a variety of vertebrate tissues. Examples include muscle stem cells ((Bjornson et al., 2012); (Mourikis et al., 2012)), neural stem cells (e.g., (Ehm et al., 2010); (Imayoshi et al., 2010)), and intestinal stem cells ((Pellegrinet et al., 2011)). The control of C. elegans GSCs by the DTC may therefore provide a simple paradigm for a widely used mechanism of stem cell control.
3.2. Specification of the Distal Tip Cell to its niche-forming fate
Given that GSCs are maintained by their proximity to the DTC, molecular specification of the DTC's capacity to form a niche is central to stem cell regulation. The DTC is born in the invariant cell lineage of the somatic gonad ((Kimble et al., 1979)), and its fate is specified through an asymmetric cell division controlled by the Wnt/β-catenin asymmetry pathway ((Kidd et al., 2005); (Phillips et al., 2007); (Siegfried et al., 2002)). In the daughter cell destined to become the DTC, the Wnt/β-catenin asymmetry pathway activates transcription of ceh-22, which encodes the single C. elegans Nkx2.5 homeodomain transcription factor ((Lam et al., 2006)). When either β-catenin or ceh-22 is absent, both daughters are transformed to another cell type of the somatic gonad; conversely, when either transcription factor is aberrantly expressed in both daughters, they both become DTCs and form ectopic niches. Therefore, the Wnt/β-catenin asymmetry pathway and its Nkx2.5 target specify the DTC and its niche fate.
3.3. FBF regulates the differentiation state of adult GSC stem cells
Downstream of GLP-1/Notch signaling is an intrinsically-acting regulator of GSCs: FBF, the collective term for two nearly identical PUF (for Pumilio and FBF) RNA-binding proteins, FBF-1 and FBF-2 ((Zhang et al., 1997)). Removal of either FBF paralog individually has little effect on GSCs, but removal of both FBF-1 and FBF-2 together causes a complete failure in maintenance of adult GSCs: in fbf-1 fbf-2 adults, GSCs fail to self-renew and instead enter the meiotic cell cycle and differentiate ((Crittenden et al., 2002); (Lamont et al., 2004)).
Like all PUF proteins, FBF is a sequence-specific RNA-binding protein that binds a well-defined regulatory element, typically within the 3′ untranslated region of its target mRNAs (Fig. 5) ((Bernstein et al., 2005); (Zhang et al., 1997)). The major mode of mRNA regulation by PUF is repression ((Wickens et al., 2002)). One mechanism for mRNA repression, verified in yeast, worms, flies, and mammals, involves PUF recruitment of the CCR4-Not complex, a deadenylase that can either destabilize the mRNA or lead to its silencing (e.g. (Goldstrohm et al., 2008)). A second mechanism, validated so far in worms and mammals, involves PUF recruitment of an Argonaute protein and a core component of the translational machinery, eEF1-alpha, to block translational elongation ((Friend et al., 2012)). Other mechanisms may also exist, given that PUF proteins interact with additional protein partners, although the biochemical significance of those interactions remains poorly understood. Finally, FBF is also capable of positively regulating at least two of its targets, via interaction with the GLD-2 poly(A) polymerase ((Kaye et al., 2009); (Suh et al., 2009)).
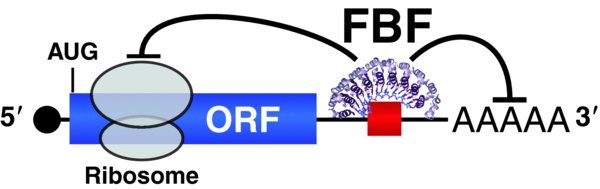
Figure 5. FBF post-transcriptional repression maintains adult GSCs. FBF can silence mRNAs either by regulating translational elongation or poly(A) tail length. FBF target mRNAs encode many differentiation regulators. The FBF target mRNA includes a 5′ cap, black; open reading frame (ORF), blue; and 3′ untranslated region (3′UTR) with a poly(A) tail (AAAAA). FBF is represented by the crystal structure of its RNA binding region ((Wang et al., 2009)); FBF binds to a FBF binding element, red, which is typically located in the 3′UTR. A ribosome is schematized during translational elongation; diagram is not to scale.
Identification of FBF target mRNAs reveals an elaborate regulatory network controlling self-renewal vs. differentiation (Fig. 6). All of the targets identified using candidate approaches were found to encode proteins regulating germ cell differentiation (e.g. GLD proteins promote meiotic entry, FOG-1 protein promotes the sperm fate). Putative targets identified using a genomic approach include >1000 additional mRNAs, many of which also encode key regulators of differentiation ((Kershner et al., 2013); (Kershner et al., 2010)). Thus, FBF emerges as a broad-spectrum regulator of likely >1000 mRNAs.
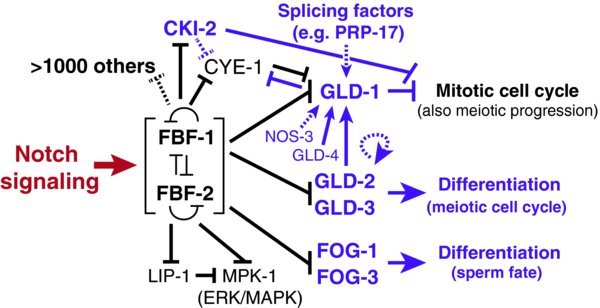
Figure 6. RNA regulatory network for GSC self-renewal and differentiation. FBF serves as a major a hub in the network controlling self-renewal and differentiation. FBF inhibits expression of many differentiation regulators. Some FBF targets control entry into the meiotic cell cycle and others control the sperm/oocyte decision. In addition, FBF inhibits MPK-1, which encodes the major C. elegans ERK/MAP kinase ((Lackner et al., 1994); (Wu et al., 1994)). FBF also likely regulates over 1000 additional mRNAs, identified by FBF immunoprecipitation followed by microarray analysis of associated mRNAs ((Kershner et al., 2010)), many of which are shared as likely human Pum targets (see text).
Importantly, PUF proteins are conserved stem cell regulators. Not only does FBF regulate C. elegans GSCs, but the PUF protein Pumilio similarly regulates adult GSCs in Drosophila ((Forbes et al., 1998); (Lin et al., 1997)), and planarian DjPum maintains neoblasts, the magical cells responsible for planarian regeneration ((Salvetti et al., 2005)). The role of PUF proteins in controlling vertebrate stem cells is not yet clear.
Given their conservation as stem cell regulators, the question emerges: Do PUF proteins across organisms regulate stem cells through a set of common target mRNAs? The answer is not yet known, but a comparison of putative PUF target mRNAs from C. elegans (FBF in GSCs) and humans (PUM in immortalized cells) reveals significant overlap ((Kershner et al., 2013); (Kershner et al., 2010)). The common target mRNAs encode components of major developmental signaling pathways, as well as cell cycle and cell death regulators (e.g. components of Ras/MAPK, Notch, PI3/Akt, Hedgehog, Wnt pathways plus cyclin E, cyclin B and CED-4). Even so, only one common PUF target mRNA, that encoding ERK/MAP kinase, has been validated to date in both C. elegans and human embryonic stem cells ((Lee et al., 2007)). Considerable work remains to analyze the significance of other common PUF targets, but these initial findings support the idea that PUF proteins are broad-spectrum inhibitors of differentiation throughout the animal kingdom.
3.4. Nutrition regulates GSC self-renewal and germline regeneration
The GSC regulation described above dominates in well-fed animals grown under optimal laboratory conditions. However, additional controls come into play when food is scarce. When first-stage larvae (L1s) are hatched into nutrient-poor conditions, they enter a phase of L1 diapause, during which all cell divisions arrest, including GSC divisions; GSC divisions also arrest during the dauer larval stage, a stress-resistant, alternative third larval stage that forms in response to low food and crowding. During both L1 diapause and the dauer stage, GSCs arrest in the G2/M stage of the cell cycle ((Fukuyama et al., 2006); (Narbonne et al., 2006)). This quiescence is regulated by the tumor suppressor gene daf-18/PTEN and AMP-activated protein kinase ((Fukuyama et al., 2006); (Fukuyama et al., 2012); (Narbonne et al., 2006)). In addition to L1 diapause and the dauer stage, germline proliferation is also sensitive to dietary restriction during the third and fourth larval stages (L3 and L4). This later effect is controlled in two ways: Insulin/IGF-like signaling and ribosomal protein S6 kinase act in the germline to control cell-cycle progression ((Korta et al., 2012); (Michaelson et al., 2010)), and the TGF-ß pathway acts in the DTC to alter the balance between GSC proliferation and differentiation ((Dalfó et al., 2012)). Finally, when adult animals are starved, adult GSCs cease dividing ((Salinas et al., 2006)). In adults starved for longer periods (i.e. starvation beginning in L4s and extending into adulthood), growth is arrested overall, and oocyte production is dramatically slowed ((Angelo et al., 2009); (Seidel et al., 2011)). The germlines of these animals shrink over time and can ultimately contain as few as 35 germ cells per gonadal arm (compared to ∼1,000 per gonadal arm in a well-fed adult). Upon re-feeding, these germ cells can regenerate a full germline tissue comparable to that of an adult that had never experienced starvation ((Angelo et al., 2009); (Seidel et al., 2011)). Thus, GSCs respond to changes in food availability throughout the life of the animal, although the mechanisms regulating this response in adults are less well understood.
4. Molecular regulation of GSC differentiation
When GSC daughter cells leave their niche, they enter the meiotic cell cycle and differentiate as either sperm or oocyte. C. elegans gamete production is unusual in that hermaphrodites first produce sperm as larvae and later make oocytes as adults. Yet gamete production in adults is similar to that of most species – adult C. elegans males make sperm and adult C. elegans hermaphrodites make oocytes.
4.1. Regulation of the mitosis/meiosis cell cycle decision
Germ cells can divide either mitotically or meiotically. Mitotic germ cell divisions expand germ cell number during development and maintain it in adults, whereas the meiotic cell cycle generates haploid gametes, typically after chromosomal recombination. The balance between these cycles must be regulated to maintain GSCs but prevent tumor formation. For simplicity, this cell cycle regulation is dubbed the “mitosis-meiosis decision”.
One confusion has been that the mitosis-meiosis decision is sometimes equated with the decision between self-renewal and differentiation. Yet the ∼225 mitotically dividing germ cells include one small pool of ∼50 undifferentiated or naïve cells within the niche (the GSC pool) and another larger and more proximal pool of ∼150 cells that have been triggered to progress towards differentiation ((Cinquin et al., 2010)). Therefore the two decisions are not identical: the mitosis-meiosis decision focuses on cell cycle regulation while the self-renewal vs. differentiation decision focuses on regulation of the differentiation state — with overt meiotic entry being only one readout of that differentiated state.
Notch signaling maintains C. elegans germ cells in the mitotic cell cycle throughout development and adulthood, and the FBF RNA-binding protein maintains germ cells in this cycle in late larvae and adults (see above). FBF promotes the mitotic cell cycle by repressing several mRNA targets: cki-2 mRNA, which encodes a mitotic cell cycle inhibitor ((Kalchhauser et al., 2011)); gld-1 and gld-3 mRNAs, which encode proteins that drive meiotic entry ((Crittenden et al., 2002); (Eckmann et al., 2004)); and mRNAs encoding the functional machinery of the meiotic program (e.g. synaptonemal complex proteins) ((Merritt et al., 2010)). Genomic analyses have identified many more likely targets of FBF with similar functions ((Kershner et al., 2010)).
The key regulators of meiotic entry include three GLD (for GermLine development Defective) proteins ((Eckmann et al., 2004); (Eckmann et al., 2002); (Francis et al., 1995); (Kadyk et al., 1998); (Wang et al., 2002)). Details of regulation by the GLD proteins can be found elsewhere ((Kimble et al., 2011)), but we emphasize that like the two FBF proteins, the three GLD proteins all regulate mRNAs. GLD-1 is a sequence-specific RNA-binding protein and translational repressor ((Jan et al., 1999); (Lee et al., 2010)). GLD-1 targets have been studied intensively and include mRNAs encoding the GLP-1/Notch receptor and cyclin E ((Biedermann et al., 2009); (Jungkamp et al., 2011); (Lee et al., 2001); (Marin et al., 2003); (Wright et al., 2011)). By contrast, GLD-2 and its partner GLD-3 form a heterodimeric poly(A) polymerase that activates translation ((Suh et al., 2006); (Wang et al., 2002)). Therefore, the major regulators of the mitosis-meiosis decision (FBF and the GLDs) function post-transcriptionally – FBF and GLD-1 by repression and GLD-2 and GLD-3 by activation.
4.2. Regulation of the sperm/oocyte cell fate decision
Normally, adult hermaphrodites make only oocytes, whereas adult males make only sperm. Germline sex is determined by a global sex determining pathway that relies on cell signaling and germline-specific regulators that act within the germ cell (reviewed in (Ellis et al., 1995)). Details of sperm-oocyte fate specification are beyond the scope of this chapter, but here we emphasize the major regulators that act downstream of cell signaling to control the sperm/oocyte decision within germ cells.
The germline-specific regulators of the sperm/oocyte decision include FOG-1 and FOG-3, which act at the end of the sex determination pathway to drive specification of the sperm fate ((Barton et al., 1990); (Ellis et al., 1995)). Loss of either FOG-1 or FOG-3 leads to germline sexual transformation from sperm to oocyte production. Importantly, FOG-1 and FOG-3 also regulate sperm specification in C. remanei, a closely related male/female species ((Chen et al., 2001); (Cho, S. 2003Hermaphrodite or Female: Rapid evolution of mating systems in the genus CaenorhabditisDissertation, The University of Michigan (Molecular, Cellular, and Developmental Biology).)). Therefore, although the regulatory machinery enabling sperm production in XX hermaphrodites has evolved recently ((Ellis et al., 2007); (Haag et al., 2013)), the terminal regulators driving the sperm fate are conserved and likely ancient.
Like the major regulators of the mitosis-meiosis decision, primary regulators of the sperm/oocyte decision regulate mRNAs. FOG-1 is a CPEB RNA-binding protein ((Jin et al., 2001); (Luitjens et al., 2000)), and FOG-3 is a Tob/BTG homolog ((Chen et al., 2000)). Oocyte-promoting regulators include GLD-1, a quaking/STAR RNA-binding protein ((Jones et al., 1996); (Kim et al., 2009)), and RNP-8, a novel RRM motif-containing protein ((Kim et al., 2009)). Thus, unlike in somatic cells (e.g. muscle, nerve), where cell fate is predominantly controlled by transcription factors, the primary cell fate regulators in germ cells act post-transcriptionally.
Remarkably, the sperm/oocyte decision is made continuously (or must be maintained) even in adulthood. When either FOG-1 or FOG-3 is depleted during adulthood, using RNAi, the adult germline switches from sperm to oocyte production (e.g. (Chen et al., 2000)). Likewise, addition of a sperm-promoting regulator, accomplished using a temperature-sensitive gain-of-function mutant, can switch adult hermaphrodite germlines from oocyte to sperm production ((Barton et al., 1987)). Therefore, even in adults, some germ cells are not irreversibly committed to either sperm or oocyte fate. Of particular interest to this chapter, the GSCs remain labile while their daughters become committed to either the sperm or oocyte fate soon after they leave their niche and transition into the meiotic cell cycle ((Morgan et al., 2013)). Thus, as expected for stem cell multipotency, GSCs retain potential to generate either sperm or oocytes.
4.3. Relationship of mitosis/meiosis and sperm/oocyte decisions
A major question in the germ cell field has been whether the mitosis/meiosis decision and the sperm/oocyte decision are one and the same ((Kimble et al., 2007)). In C. elegans, these decisions have now been separated definitively: using genetic and chemical tools to manipulate these decisions separately, germ cells initially fated to become sperm can be induced to switch to an oocyte fate even after meiotic entry has occurred ((Morgan et al., 2013)). Nonetheless, C. elegans GSC progeny progress towards both decisions at about the same time, and the regulatory networks driving the two decisions overlap ((Kimble et al., 2007); (Morgan et al., 2013)). Thus, the two decisions are separate but linked. Given that germ cells are an ancient cell type, we suggest that the two decisions may be distinct in all organisms, with the linkage between them more or less elaborate in individual species. Teasing apart these decisions was not simple in C. elegans and may prove difficult in less tractable organisms.
4.4. Regulation of germ cell totipotency
GSCs normally make sperm or oocytes, which ultimately fuse and contribute to all cell types in the next generation. Thus, germ cells are specialized for their potential to differentiate as any cell type, a phenomenon called totipotency ((Seydoux et al., 2006)). An understanding of germ cell totipotency may inform our understanding of pluripotency and directed somatic differentiation.
One regulator of C. elegans germ cell totipotency is transcriptional: germ cells can be converted into specific neurons after ectopic expression of a single transcription factor dedicated to specification of that neuron, coupled with deacetylase inhibition ((Tursun et al., 2011)). A different and more unusual regulator of totipotency is post-transcriptional ((Ciosk et al., 2006)). C. elegans germ cells can be transformed into neuronal, muscle or intestinal cells simply by removing a single RNA-binding protein (GLD-1), an effect that is enhanced dramatically by removing two RNA-binding proteins (GLD-1 and MEX-3). Intriguingly, the transcriptional and post-transcriptional germ cell conversions into somatic cells occur in different parts of the germline tissue. The transcriptional conversion occurs in mitotically dividing germ cells in the distal gonad, whereas the post-transcriptional conversion occurs more proximally in pachytene germ cells ((Ciosk et al., 2006); (Tursun et al., 2011)). Consistent with this spatial distinction, proximal germ cells rely largely on post-transcriptional regulation (e.g. meiotic entry), whereas GSCs in the distal gonad are regulated both transcriptionally (e.g. Notch signaling) and post-transcriptionally (e.g. FBF). The post-transcriptional regulation of totipotency in the C. elegans germline may foreshadow the existence of a similar mechanism in vertebrate cells.
5. Conclusions, perspectives and future challenges
C. elegans GSCs stand out as a model for stem cell regulation that relies on localized niche signaling rather than regulated asymmetric cell divisions. Their well-defined niche provides a paradigm for stem cell regulation by Notch signaling, and the network downstream of Notch signaling provides a paradigm for post-transcriptional regulation of self-renewal and cell fate specification. Thus, key transcriptional and post-transcriptional mechanisms work together to maintain GSCs and regulate their decision between self-renewal and differentiation. Intriguingly, transcriptional and post-transcriptional mechanisms also control totipotency in the C. elegans germline. We suggest that a combination of transcriptional and post-transcriptional controls is critical not only for controlling GSCs but also more broadly for other totipotent or pluripotent cells to achieve ultimate plasticity.
Although much is known about C. elegans GSCs and their regulation, many fundamental questions remain. What defines a stem cell in a system without regulated asymmetric divisions? Evidence for a pool of 35-70 GSCs is strong, but what determines that number and what molecular landscape defines their stem cell state? Evidence for a network regulating the balance between self-renewal and differentiation is also strong, but what regulates the balance and the dynamics of the shift from self-renewal to differentiation? What regulatory mechanisms maintain GSC totipotency and how are GSCs channeled into gamete production without a loss in totipotency? How can a GSC pool regenerate a functional germline of the correct size and organization? The foundation has been laid for addressing these basic questions, and recent advances in genome editing (e.g. (Friedland et al., 2013); (Golic et al., 2013)) make them yet more tractable in this already powerful system.
Acknowledgments
We thank Anne Helsley-Marchbanks and Laura Vanderploeg for help with manuscript preparation and figures. HS is an Ellison Medical Foundation Fellow of the Life Sciences Research Foundation. JK is supported by NIH (GM069454) and is an investigator of the Howard Hughes Medical Institute.
Copyright: © 2013 Judith Kimble and Hannah Seidel
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
* Corresponding author: HHMI/Department of Biochemistry, 433 Babcock Drive, Madison, WI 53706-1544, Phone: 608-262-6188, Fax: 608-265-5820, E-mail: jekimble@wisc.edu
Edited by Patricia Donahoe and Haifan Lin. Last revised October 31, 2013. Published November 15, 2013. This chapter should be cited as: Kimble, J. and Seidel, H., C. elegans germline stem cells and their niche (November 15, 2013), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.95.1.
References
- Angelo, G. Van Gilst, M.R. (2009). Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326, 954–958. Article Abstract Abstract DOI
- Austin, J. Kimble, J. (1987). glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51, 589–599. Article Abstract Abstract DOI
- Barton, M.K. Schedl, T.B. Kimble, J. (1987). Gain-of-function mutations of fem-3, a sex-determination gene in Caenorhabditis elegans. Genetics 115, 107–119. Abstract Abstract
- Barton, M.K. Kimble, J. (1990). fog-1, a regulatory gene required for specification of spermatogenesis in the germ line of Caenorhabditis elegans. Genetics 125, 29–39. Abstract Abstract
- Bernstein, D. Hook, B. Hajarnavis, A. Opperman, L. Wickens, M. (2005). Binding specificity and mRNA targets of a C. elegans PUF protein, FBF-1. RNA 11, 447–458. Article Abstract Abstract DOI
- Berry, L.W. Westlund, B. Schedl, T. (1997). Germ-line tumor formation caused by activation of glp-1, a Caenorhabditis elegans member of the Notch family of receptors. Development 124, 925–936. Abstract Abstract
- Biedermann, B. Wright, J. Senften, M. Kalchhauser, I. Sarathy, G. Lee, M.H. Ciosk, R. (2009). Translational repression of cyclin E prevents precocious mitosis and embryonic gene activation during C. elegans meiosis. Dev Cell 17, 355–364. Article Abstract Abstract DOI
- Bjornson, C.R. Cheung, T.H. Liu, L. Tripathi, P.V. Steeper, K.M. Rando, T.A. (2012). Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30, 232–242. Article Abstract Abstract DOI
- Chen, P.-J. Singal, A. Kimble, J. Ellis, R.E. (2000). A novel member of the Tob family of proteins controls sexual fate in Caenorhabditis elegans germ cells. Dev Biol 217, 77–90. Article Abstract Abstract DOI
- Chen, P.-J. Cho, S. Jin, S.-W. Ellis, R.E. (2001). Specification of germ cell fates by FOG-3 has been conserved during nematode evolution. Genetics 158, 1513–1525. Abstract Abstract
- Cho, S. 2003Hermaphrodite or Female: Rapid evolution of mating systems in the genus CaenorhabditisDissertation, The University of Michigan (Molecular, Cellular, and Developmental Biology).
- Christensen, S. Kodoyianni, V. Bosenberg, M. Friedman, L. Kimble, J. (1996). lag-1, a gene required for lin-12 and glp-1 signaling in Caenorhabditis elegans, is homologous to human CBF1 and Drosophila Su(H). Development 122, 1373–1383. Abstract Abstract
- Cinquin, O. Crittenden, S.L. Morgan, D.E. Kimble, J. (2010). Progression from a stem cell-like state to early differentiation in the C. elegans germ line. Proc Natl Acad Sci USA 107, 2048–2053. Article Abstract Abstract DOI
- Ciosk, R. DePalma, M. Priess, J.R. (2006). Translational regulators maintain totipotency in the Caenorhabditis elegans germline. Science 311, 851–853. Article Abstract Abstract DOI
- Crittenden, S.L. Troemel, E.R. Evans, T.C. Kimble, J. (1994). GLP-1 is localized to the mitotic region of the C. elegans germ line. Development 120, 2901–2911. Abstract Abstract
- Crittenden, S.L. Bernstein, D.S. Bachorik, J.L. Thompson, B.E. Gallegos, M. Petcherski, A.G. Moulder, G. Barstead, R. Wickens, M. Kimble, J. (2002). A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417, 660–663. Article Abstract Abstract DOI
- Crittenden, S.L. Leonhard, K.A. Byrd, D.T. Kimble, J. (2006). Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell 17, 3051–3061. Article Abstract Abstract DOI
- Dalfó, D. Michaelson, D. Hubbard, E.J. (2012). Sensory regulation of the C. elegans germline through TGF-β-dependent signaling in the niche. Curr Biol 22, 712–719. Abstract Abstract
- Doyle, T.G. Wen, C. Greenwald, I. (2000). SEL-8, a nuclear protein required for LIN-12 and GLP-1 signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA 97, 7877–7881. Article Abstract Abstract DOI
- Eckmann, C.R. Kraemer, B. Wickens, M. Kimble, J. (2002). GLD-3, a Bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev Cell 3, 697–710. Article Abstract Abstract DOI
- Eckmann, C.R. Crittenden, S.L. Suh, N. Kimble, J. (2004). GLD-3 and control of the mitosis/meiosis decision in the germline of Caenorhabditis elegans. Genetics 168, 147–160. Article Abstract Abstract DOI
- Ehm, O. Goritz, C. Covic, M. Schaffner, I. Schwarz, T.J. Karaca, E. Kempkes, B. Kremmer, E. Pfrieger, F.W. Espinosa, L. et al. (2010). RBPJκ-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci 30, 13794–13807. Article Abstract Abstract DOI
- Ellis, R.E. Kimble, J. (1995). The fog-3 gene and regulation of cell fate in the germ line of Caenorhabditis elegans. Genetics 139, 561–577. Abstract Abstract
- Ellis, R. Schedl, T. (2007). Sex determination in the germ line. In WormBook, The C. elegans Research Community, ed. (WormBook). Abstract Abstract
- Forbes, A. Lehmann, R. (1998). Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125, 679–690. Abstract Abstract
- Francis, R. Barton, M.K. Kimble, J. Schedl, T. (1995). gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606. Abstract Abstract
- Friedland, A.E. Tzur, Y.B. Esvelt, K.M. Colaiacovo, M.P. Church, G.M. Calarco, J.A. (2013). Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods 10, 741–743. Article Abstract Abstract DOI
- Friend, K. Campbell, Z.T. Cooke, A. Kroll-Conner, P. Wickens, M.P. Kimble, J. (2012). A conserved PUF–Ago–eEF1A complex attenuates translation elongation. Nat Struct Mol Biol 19, 176–183. Article Abstract Abstract DOI
- Fukuyama, M. Rougvie, A.E. Rothman, J.H. (2006). C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr Biol 16, 773–779. Article Abstract Abstract DOI
- Fukuyama, M. Sakuma, K. Park, R. Kasuga, H. Nagaya, R. Atsumi, Y. Shimomura, Y. Takahashi, S. Kajiho, H. Rougvie, A. et al. (2012). C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol Open 1, 929–936. Article Abstract Abstract DOI
- Goldstrohm, A.C. Hook, B.A. Wickens, M. (2008). Regulated deadenylation in vitro. Methods Enzymol 448, 77–106. Article Abstract Abstract DOI
- Golic, K.G. (2013). RNA-guided nucleases: A new era for engineering the genomes of model and nonmodel organisms. Genetics 195, 303–308. Article Abstract Abstract DOI
- Haag, E.S. Liu, Q. (2013). Using Caenorhabditis to explore the evolution of the germ line. Adv Exp Med Biol 757, 405–425. Article Abstract Abstract DOI
- Henderson, S.T. Gao, D. Lambie, E.J. Kimble, J. (1994). lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development 120, 2913–2924. Abstract Abstract
- Imayoshi, I. Sakamoto, M. Yamaguchi, M. Mori, K. Kageyama, R. (2010). Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci 30, 3489–3498. Article Abstract Abstract DOI
- Jan, E. Motzny, C.K. Graves, L.E. Goodwin, E.B. (1999). The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J 18, 258–269. Article Abstract Abstract DOI
- Jin, S.-W. Kimble, J. Ellis, R.E. (2001). Regulation of cell fate in Caenorhabditis elegans by a novel cytoplasmic polyadenylation element binding protein. Dev Biol 229, 537–553. Article Abstract Abstract DOI
- Jones, A.R. Francis, R. Schedl, T. (1996). GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev Biol 180, 165–183. Abstract Abstract
- Jungkamp, A.C. Stoeckius, M. Mecenas, D. Grun, D. Mastrobuoni, G. Kempa, S. Rajewsky, N. (2011). In vivo and transcriptome-wide identification of RNA binding protein target sites. Mol Cell 44, 828–840. Article Abstract Abstract DOI
- Kadyk, L.C. Kimble, J. (1998). Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development 125, 1803–1813. Abstract Abstract
- Kalchhauser, I. Farley, B.M. Pauli, S. Ryder, S.P. Ciosk, R. (2011). FBF represses the Cip/Kip cell-cycle inhibitor CKI-2 to promote self-renewal of germline stem cells in C. elegans. EMBO J 30, 3823–3829. Article Abstract Abstract DOI
- Kaye, J.A. Rose, N.C. Goldsworthy, B. Goga, A. L’Etoile, N.D. (2009). A 3′UTR Pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron 61, 57–70. Article Abstract Abstract DOI
- Kershner, A.M. Kimble, J. (2010). Genome-wide analysis of mRNA targets for Caenorhabditis elegans FBF, a conserved stem cell regulator. Proc Natl Acad Sci USA 107, 3936–3941. Article Abstract Abstract DOI
- Kershner, A. Crittenden, S.L. Friend, K. Sorensen, E.B. Porter, D.F. Kimble, J. (2013). Germline stem cells and their regulation in the nematode Caenorhabditis elegans. Adv Exp Med Biol 786, 29–46. Article Abstract Abstract DOI
- Kidd, A.R. Miskowski, J.A. Siegfried, K.R. Sawa, H. Kimble, J. (2005). A β-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell 121, 761–772. Article Abstract Abstract DOI
- Kim, K.W. Nykamp, K. Suh, N. Bachorik, J.L. Wang, L. Kimble, J. (2009). Antagonism between GLD-2 binding partners controls gamete sex. Dev Cell 16, 723–733. Article Abstract Abstract DOI
- Kimble, J. Hirsh, D. (1979). The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol 70, 396–417. Article Abstract Abstract DOI
- Kimble, J.E. White, J.G. (1981). On the control of germ cell development in Caenorhabditis elegans. Dev Biol 81, 208–219. Article Abstract Abstract DOI
- Kimble, J. Crittenden, S.L. (2007). Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol 23, 405–433. Article Abstract Abstract DOI
- Kimble, J. (2011). Molecular regulation of the mitosis/meiosis decision in multicellular organisms. Cold Spring Harb Perspect Biol 3, a0002683. Article Abstract Abstract DOI
- Kopan, R. Ilagan, M.X. (2009). The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233. Article Abstract Abstract DOI
- Korta, D.Z. Tuck, S. Hubbard, E.J.A. (2012). S6K links cell fate, cell cycle and nutrient response in C. elegans germline stem/progenitor cells. Development 139, 859–870. Article Abstract Abstract DOI
- Lackner, M.R. Kornfeld, K. Miller, L.M. Horvitz, H.R. Kim, S.K. (1994). A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes Dev 8, 160–173. Article Abstract Abstract DOI
- Lam, N. Chesney, M.A. Kimble, J. (2006). Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr Biol 16, 287–295. Article Abstract Abstract DOI
- Lamont, L.B. Crittenden, S.L. Bernstein, D. Wickens, M. Kimble, J. (2004). FBF-1 and FBF-2 regulate the size of the mitotic region in the C. elegans germline. Dev Cell 7, 697–707. Article Abstract Abstract DOI
- Lee, M.-H. Schedl, T. (2001). Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev 15, 2408–2420. Article Abstract Abstract DOI
- Lee, M.-H. Hook, B. Pan, G. Kershner, A.M. Merritt, C. Seydoux, G. Thomson, J.A. Wickens, M. Kimble, J. (2007). Conserved regulation of MAP kinase expression by PUF RNA-binding proteins. PLoS Genet 3, e233. Article Abstract Abstract DOI
- Lee, M.-H. Schedl, T. (2010). C. elegans star proteins, GLD-1 and ASD-2, regulate specific RNA targets to control development. Adv Exp Med Biol 693, 106–122. Article Abstract Abstract DOI
- Lin, H. Spradling, A.C. (1997). A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124, 2463–2476. Abstract Abstract
- Luitjens, C. Gallegos, M. Kraemer, B. Kimble, J. Wickens, M. (2000). CPEB proteins control two key steps in spermatogenesis in C. elegans. Genes Dev 14, 2596–2609. Article Abstract Abstract DOI
- Marin, V.A. Evans, T.C. (2003). Translational repression of a C. elegans Notch mRNA by the STAR/KH domain protein GLD-1. Development 130, 2623–2632. Article Abstract Abstract DOI
- McGovern, M. Voutev, R. Maciejowski, J. Corsi, A.K. Hubbard, E.J. (2009). A “latent niche” mechanism for tumor initiation. Proc Natl Acad Sci USA 106, 11617–11622. Article Abstract Abstract DOI
- Merritt, C. Seydoux, G. (2010). Transgenic solutions for the germline. In WormBook, The C. elegans Research Community, ed. (WormBook). Article Abstract Abstract DOI
- Michaelson, D. Korta, D.Z. Capua, Y. Hubbard, E.J. (2010). Insulin signaling promotes germline proliferation in C. elegans. Development 137, 671–680. Article Abstract Abstract DOI
- Morgan, D.E. Crittenden, S.L. Kimble, J. (2010). The C. elegans adult male germline: Stem cells and sexual dimorphism. Dev Biol 346, 204–214. Article Abstract Abstract DOI
- Morgan, C.T. Noble, D. Kimble, J. (2013). Mitosis-meiosis and sperm-oocyte fate decisions are separable regulatory events. Proc Natl Acad Sci USA 110, 3411–3416. Article Abstract Abstract DOI
- Mourikis, P. Sambasivan, R. Castel, D. Rocheteau, P. Bizzarro, V. Tajbakhsh, S. (2012). A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30, 243–252. Article Abstract Abstract DOI
- Nadarajan, S. Govindan, J.A. McGovern, M. Hubbard, E.J.A. Greenstein, D. (2009). MSP and GLP-1/Notch signaling coordinately regulate actomyosin-dependent cytoplasmic streaming and oocyte growth in C. elegans. Development 136, 2223–2234. Article Abstract Abstract DOI
- Narbonne, P. Roy, R. (2006). Inhibition of germline proliferation during C. elegans dauer development requires PTEN, LKB1 and AMPK signalling. Development 133, 611–619. Article Abstract Abstract DOI
- Pellegrinet, L. Rodilla, V. Liu, Z. Chen, S. Koch, U. Espinosa, L. Kaestner, K.H. Kopan, R. Lewis, J. Radtke, F. (2011). Dll1- and Dll4-mediated Notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140, 1230-1240 e1231-1237. Article Abstract Abstract DOI
- Petcherski, A.G. Kimble, J. (2000). LAG-3 is a putative transcriptional activator in the C. elegans Notch pathway. Nature 405, 364–368. Abstract Abstract
- Phillips, B.T. Kidd, III, A.R. King, R. Hardin, J. Kimble, J. (2007). Reciprocal asymmetry of SYS-1/β-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci USA 104, 3231–3236. Article Abstract Abstract DOI
- Salinas, L.S. Maldonado, E. Navarro, R.E. (2006). Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ 13, 2129–2139. Article Abstract Abstract DOI
- Salvetti, A. Rossi, L. Lena, A. Batistoni, R. Deri, P. Rainaldi, G. Locci, M.T. Evangelista, M. Gremigni, V. (2005). DjPum, a homologue of Drosophila Pumilio, is essential to planarian stem cell maintenance. Development 132, 1863–1874. Article Abstract Abstract DOI
- Seidel, H.S. Kimble, J. (2011). The oogenic germline starvation response in C. elegans. PLoS ONE 6, e28074. Article Abstract Abstract DOI
- Seydoux, G. Braun, R.E. (2006). Pathway to totipotency: lessons from germ cells. Cell 127, 891–904. Article Abstract Abstract DOI
- Siegfried, K. Kimble, J. (2002). POP-1 controls axis formation during early gonadogenesis in C. elegans. Development 129, 443–453. Abstract Abstract
- Suh, N. Jedamzik, B. Eckmann, C.R. Wickens, M. Kimble, J. (2006). The GLD-2 poly(A) polymerase activates gld-1 mRNA in the Caenorhabditis elegans germ line. Proc Natl Acad Sci USA 103, 15108–15112. Article Abstract Abstract DOI
- Suh, N. Crittenden, S.L. Goldstrohm, A.C. Hook, B. Thompson, B. Wickens, M. Kimble, J. (2009). FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 181, 1249–1260. Article Abstract Abstract DOI
- Sulston, J.E. Horvitz, H.R. (1977). Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56, 110–156. Article Abstract Abstract DOI
- Sulston, J.E. Schierenberg, E. White, J.G. Thomson, J.N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100, 64–119. Article Abstract Abstract DOI
- Tax, F.E. Yeargers, J.J. Thomas, J.H. (1994). Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature 368, 150–154. Article Abstract Abstract DOI
- Tursun, B. Patel, T. Kratsios, P. Hobert, O. (2011). Direct conversion of C. elegans germ cells into specific neuron types. Science 331, 304–308. Article Abstract Abstract DOI
- Wang, L. Eckmann, C.R. Kadyk, L.C. Wickens, M. Kimble, J. (2002). A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419, 312–316. Article Abstract Abstract DOI
- Wang, Y. Opperman, L. Wickens, M. Hall, T.M. (2009). Structural basis for specific recognition of multiple mRNA targets by a PUF regulatory protein. Proc Natl Acad Sci USA 106, 20186–20191. Article Abstract Abstract DOI
- Wickens, M. Bernstein, D.S. Kimble, J. Parker, R. (2002). A PUF family portrait: 3’UTR regulation as a way of life. Trends Genet 18, 150–157. Article Abstract Abstract DOI
- Wright, J.E. Gaidatzis, D. Senften, M. Farley, B.M. Westhof, E. Ryder, S.P. Ciosk, R. (2011). A quantitative RNA code for mRNA target selection by the germline fate determinant GLD-1. EMBO J 30, 533–545. Article Abstract Abstract DOI
- Wu, Y. Han, M. (1994). Suppression of activated Let-60 Ras protein defines a role of Caenorhabditis elegans Sur-1 MAP kinase in vulval differentiation. Genes Dev 8, 147–159. Article Abstract Abstract DOI
- Zhang, B. Gallegos, M. Puoti, A. Durkin, E. Fields, S. Kimble, J. Wickens, M.P. (1997). A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390, 477–484. Abstract Abstract