1. Introduction:
This protocol was developed in the Salk STEM Cell Core to enable the formation of uniform and large embryoid bodies (EBs) from pluripotent human stem cells. It assumes the cells have previously been cultured on Matrigel and typically requires 1–2 wells of a fairly confluent 6-well plate as starting material. This is a low cost alternative to commercial products like the AggreWell that has proven especially useful for the formation of larger sized EBs that show good potential for differentiation to all 3 germ layers
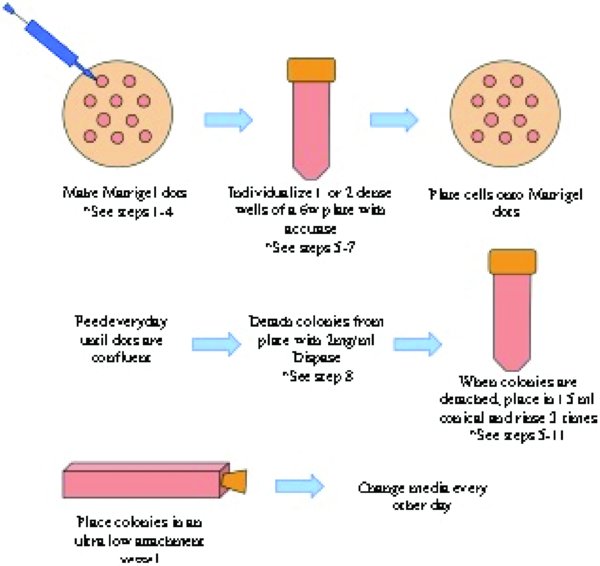
3. Protocol Steps:
3.1. Prepare the Matrigel Dots:
-
Thaw Matrigel on ice. Read the product specification sheet to determine the protein concentration of the lot of Matrigel. Transfer 0.1 mg of matrigel into 1 ml of ice-cold DMEM/F12 in a 1.5–2 ml tube, mix with a pipet. [Alternatively, use the same amount of Matrigel (un-diluted) that you would normally use for 1 well of a 6-well plate or 1/6 of a 10 cm dish, and dilute into 1 ml of ice-cold DMEM/F12]. Keep the 1 ml Matrigel solution in a small tube on ice. This needs to remain cold, as it will take about 10–15 minutes to plate out the matrigel dots.
-
Use a 0.5 to 2 ul micropipette, or preferably an electronic-pipette capable of repeat dispensing of 2 ul volumes, to make the matrigel dots. Draw up ice cold Matrigel solution into the pipet, expel out 2 ul so you can see the solution hanging on to the end of the tip, and carefully touch the solution, but not the tip of the pipette, to the 10 cm plate. The drip from the pipette will make a small dot on the plate. [Tip: place the pipet tips in the −20∘C freezer to pre-chill before using].
-
Repeat this until you have at least 50 dots on the plate. The more dots you have the higher yield you will have. We suggest a dot spacing of roughly 3–6 mm.
-
Incubate plate at 37∘C for at least 20 minutes up to 1 hour. Do not let the plate incubate overnight (the dots will dry out and new plate will have to be made). [Tip: If the drying out of Matrigel dots becomes a continuous problem, try placing numerous drops of sterile water on the inside of the lid – similar to the hanging drop EB method to increase the humidity inside the dish].
3.2. Individualize and passage the cells onto the Matrigel Dots:
-
5. Starting with 1–2 dense wells (6-well format) of hES/iPS cells (∼2–4 million cells). Rinse well with 1 ml PBS −/−. Add 1 ml Accutase (or TrypLE) for 5 to 7 minutes at 37∘C incubator until cells detach.
-
6. Add 10 mls of mTeSR1 + Rock Inhibitor to the 10 cm plates of Matrigel dots. Note: There is no need to wash or rinse the dish of Matrigel dots prior to plating cells.
-
7. Collect cells and transfer to 15 ml conical tube. Rinse well twice with 1 ml warm mTeSR1 or DMEM/F12 and add these rinses to the 15 ml conical containing the cells. Pellet cells at 200×g (or 1000 rpm in a clinical centrifuge) for 5 minutes. Aspirate the supernatant. Re-suspend the cell pellet in 5 mls mTeSR1 + Rock Inhibitor. Count the cells. Plate between 1.5–2.5 million cells on the dish of Matrigel dots.
-
8. Feed and observe daily with mTeSR1 (or medium of choice without Rock Inhibitor). In about a week, the dots will be confluent and ready to be made into EBs.
3.3. Detach intact colonies and make into EBs:
-
9. Aspirate spent media from plate. Add 4 mls to a 10 cm dish of freshly made 2 mg/ml dispase solution (pre-warmed to 37∘C.). Place in 37∘C incubator. Dissociation of colonies from the Matrigel dots can take anywhere from 15 to 30 minutes. After 15 minutes, observe under microscope every 3 to 5 minutes.
-
10. Once colonies are completely detached from the plate, gently add 5 mls warm EB medium and slowly transfer the entire solution to a 15 ml conical using at 5 ml or 10 ml pipet. Rinse the plate gently with 4 more mls warm EB media and place this into the conical. Note: It is very important to be very gentle to try to keep the colonies as intact as possible.
-
11. Wait 2 to 3 minutes for colonies to settle into the bottom of the conical. Gently aspirate supernatant, leaving about 0.5 ml of solution. Note: it is recommended to do the aspiration using a serological pipet as the large colonies are in a loose pellet at the bottom of the conical.
-
12. Wash by adding 5 mls of EB media, gently. Wait 1 to 3 minutes for colonies to settle to the bottom of the conical. Gently aspirate supernatant. Repeat the wash 3 times.
-
13. Re-suspend the colonies in 3 mls EB media. Very slowly dispense colonies into an ultra low attachment T25 flask (target 8 mls final volume), an ultra low attachment 6 well plate (target 4 mls/well final volume), or a low attachment 10 cm petri dish (target 10 mls final volume). Rinse conical with additional volume of EB medium to make the target volume for the ultra low attachment culture vessel, and add this to the flask/plate/dish. Place flask/plate/dish into 37∘C degree incubator. Change media every other day with fresh EB medium.
3.4. To change media:
Tilt flask/plate/dish and allow EBs to settle to one side. Using a 5 or 10 ml pipet, slowly remove at least 90% of media from the low attachment vessel. Gently add appropriate target volume of warm EB media (4–8 mls/T25 flask, 10 mls/10 cm Petri dish, or 2 ml/well of a 6 well). Place plate back into incubator.
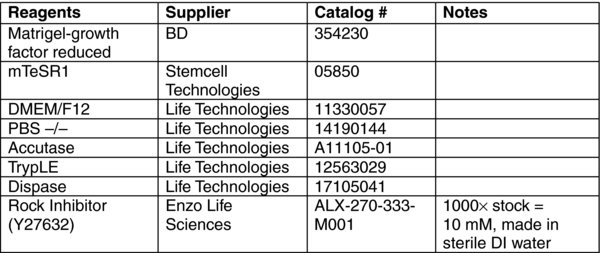
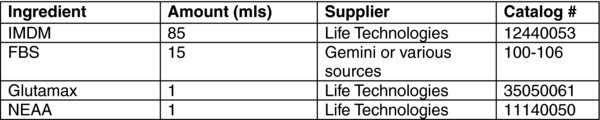
4. Materials:
Notes: Initially this protocol was developed using hES/iPS cells growing on mTeSR1 or an in-house version of this medium. It is possible, even likely, that it would work well using other feeder-free stem cell media. Be careful not to physically touch the pipet tip to the dish when spotting the Matrigel dots. Touching off typically results in smeared dots. Try to make at least 50 dots per 10 cm dish.
Acknowledgements:
This protocol was developed by W. Travis Berggren and Margaret K. Lutz of the Salk Institute Stem Cell Core Facility.
Last revised March 28, 2012. Published June 10, 2012. This chapter should be cited as: Lutz, M. and Berggren, T., Formation of embryoid bodies from Matrigel dots protocol (June 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.59.1, https://www.stembook.org.