ES cell-derived tissues exhibit a degree of immune privilege and can be naturally accepted across weak histocompatibility mismatches without any added immunosuppression. This is, in part, due to the lack of donor dendritic cells (DC) which would directly stimulate host T cell responses. In addition to this, there can be recruitment and/or induction of Treg which suppress immune attack against the tissue. This might explain why ES cell-derived tissues are so amenable to tolerance induction protocols, such as co-receptor blockade with monoclonal antibodies, when compared to skin grafts. Co-receptor blockade also appears to enhance the protective effect of regulatory T-cells
1. Introduction
The ability of embryonic stem (ES) cells to self-renew and differentiate into all somatic cell types for regenerative medicine has attracted much attention not only among the scientific community but also among the general public. Before moving into the clinic, however, much translational research is required to ensure the absence of teratoma formation from transformed ES cells and no transdifferentiation or reversion to undifferentiated progenitors. One of the other major challenges of using ES cells in regenerative medicine is their inevitable histoincompatibility to the recipient. Even though ES cells exhibit only low level of major histocompatibility complex (MHC) molecules, they increase their expression of MHC class-I when differentiated into specialized tissues (Drukker et al., 2002).
Our laboratory has shown that, even if all MHC loci are matched, disparities for minor histocompatibility antigens between donor and recipient are sufficient to provoke immune rejection of ES cell-derived tissues (Robertson et al., 2007). Consequently, in current practice, life-long immunosuppression would be necessary to promote acceptance of ES cell-derived grafts. Strategies to induce long-term tolerance for replacing life-long dependence on highly immunosuppressive drugs would be invaluable in this context to minimize toxic side effects and avoid opportunistic infections and/or malignancies. Nevertheless, despite being immunogenic, experience has taught us that ES cells and their differentiated derivatives can be accepted in appropriately conditioned hosts. For example, embryoid bodies (EB) form teratomas that survive indefinitely in fully allogeneic recipients treated with monoclonal antibodies (mAb) directed to T cell co-receptors, CD4 and CD8 (Robertson et al., 2007; Lui et al., 2010). Corbascio and colleagues have also shown that a cocktail of costimulation blocking reagents, including anti-CD40L, anti-LFA-1, and CTLA4-Ig, is sufficient to induce tolerance to human ES cells transplanted to an immunologically privileged site such as the testis of C57BL/6 mice (Grinnemo et al., 2008). Therefore, ES cell-derived tissues do seem to be amenable to tolerance induction.
The immune system has exploited random mutations and rearrangements within a limited set of inherited gene segments to generate diversity within the receptor repertoire for T- and B-lymphocytes. Self tolerance depends on mechanisms regulating T- and B-lymphocytes from the earliest stages when they first express self-reactive receptors in the thymus or bone marrow respectively and later when they encounter self antigens in the peripheral immune system or within the tissues themselves. This continuum of checkpoints and fail-safes minimizes harmful autoimmunity against self-antigens. By exploiting the same mechanisms, we might be able to promote long-term acceptance of allogeneic ES cell-derived tissues for cell replacement therapy.
2. Central tolerance
Reprogramming the immune system to accept allografts as though they were self tissues might be achieved through thymic re-education or creation of hematopoietic mixed chimerism (see Figure 1).
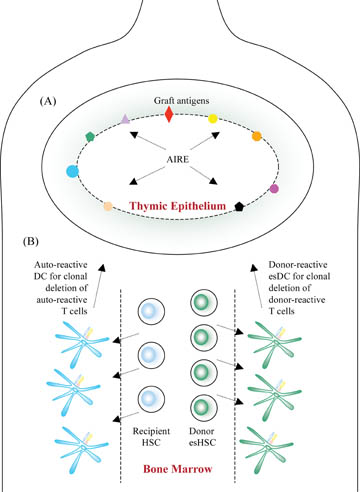
A One goal would be the regeneration of thymic epithelium by direct differentiation of ES cells into cortical and medullary thymic progenitors which express graft antigens through the action of autoimmune regulator (AIRE) for re-educating the adult immune system. B Another goal would be to aim for “Hematopoietic mixed chimerism” using a mixture of both donor ES cell-derived and recipient hematopoietic stem cells (HSC) which then generate thymic dendritic cells (DC) capable of clonal deletion of donor- and auto-reactive T cells.
2.1. Generation of ES cell-derived thymic epithelium
Many self-reactive T- and B-lymphocytes are eliminated in the primary lymphoid organs (thymus and bone marrow) to minimize autoimmunity (Dyson et al., 1991; Kappler et al., 1987; Teh et al., 1989). In fact, T-cell development in the thymus is dependent on the thymic microenvironment, in which thymic epithelial cells (TEC) are the major components (Anderson et al., 2006; Chidgey et al., 2007). The importance of TEC has been demonstrated in patients and in animals in which genetic mutations or deletions affecting TEC have dramatic effects on intrathymic T-cell development, leading to severe immunodeficiency or autoimmunity (Anderson et al., 2006; Chidgey et al., 2007; Holub et al., 1975; Rossi et al., 2007). Many of the “tissue” antigens are, contrary to expectation, ectopically (or promiscuously) expressed in the medullary thymic epithelial cells (Anderson et al., 2005; Klein et al., 1998), through the action of the AIRE (Autoimmune Regulator) gene. Natural mutations in the AIRE gene are responsible for the clinical syndrome autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), associated with widespread autoimmune disease (1997; Klein et al., 1998). Mice deficient in the AIRE gene have been shown to display a similar disease pattern (Anderson et al., 2005).
Age-dependent thymic involution, however, has been a major challenge for re-educating the immune system to accept new graft antigens given that human thymic function declines dramatically in adult life. Both cortical and medullary TEC have been shown to arise from a common progenitor (Bleul et al., 2006; Gordon et al., 2004). Recently, it has been shown that it is now possible to selectively induce development of thymic epithelial progenitors (TEP) from ES cells in vitro which might be able to address this issue (Lai and Jin, 2009). The EpCAM1+ cells derived from mouse ES cells express the phenotype of TEP. When transplanted into an irradiated thymus, implanted under the kidney capsule in vivo, these mouse ES cell-derived TEP self-renew and develop into cortical and medullary TEC. Functionally, these TEP increase thymocyte regeneration when injected directly into the thymus of lethally irradiated mice reconstituted with T-cell-depleted bone marrow. Therefore, ES cell-derived TEP can restore the thymic microenvironment and support T-cell development in vivo. If one could modulate expression of antigens on the ES cell-derived medullary TEC through regulation of the AIRE gene, one might be able to re-educate the adult immune system to accept graft antigens as self (see Figure 1A).
2.2. Generation of ES cell-derived Hematopoietic Stem Cells (HSC)
Pioneered by Ildstad and Sachs, “hematopoietic mixed chimerism” was initially achieved in animal models through complete myeloablation, a procedure which used a combination of total body irradiation and chemotherapy, and subsequent reconstitution with a mixture of both donor and recipient T cell-depleted bone marrow (Ildstad and Sachs, 1984). Both donor and recipient HSC then migrate to the recipient's bone marrow and thymus, generating thymic DC capable of clonal deletion of both auto- and donor-reactive T cells (see Figure 1B). Recently, Verda and colleagues have shown that ES cell-derived HSC (esHSC) create hematopoietic mixed chimerism and prevent autoimmune Diabetes Mellitus in NOD mice (Verda et al., 2008). Unlike HSC derived from the blood or bone marrow, esHSC are not contaminated by lymphocytes thereby mitigating the risk of GVHD. In this study, esHSC were injected either intravenously (IV) or intra bone marrow (IBM) into sublethally irradiated NOD mice. Ninety percent of mice from the IBM group and 62.5% of mice from the IV group remained normoglycemic, in contrast to the control group in which 89% of mice developed end-stage diabetes. Moreover, splenocytes from transplanted mice were found unresponsive to GAD65, a diabetes-specific autoantigen, but responded normally to third-party antigens.
Although tolerance has proven possible through hematopoietic stem cell transplants and mixed chimerism, this remains a relatively invasive procedure for promoting graft survival compared to conventional immunosuppression. Long-term mixed chimerism in mice has been demonstrated with reduced myeloablative approaches, including the use of depleting anti-CD4 and anti-CD8 mAbs, thymic irradiation and conventional immunosuppressive drugs (Nikolic et al., 2000; Tomita et al., 1994). In humans, long term mixed chimerism is hard to achieve, and the preparative regimens are still not without risk (Menendez et al., 2005).
3. esDC as a negative vaccine against graft rejection
DC are the guardians of both immunity and tolerance. They participate in various surveillance mechanisms to minimize autoimmunity. Clonal deletion by thymic DC of developing T cells, which have T cell receptors (TCR) capable of recognizing self-antigen with high affinity, contributes to the induction of self-tolerance. Autoreactive T cells with low-affinity receptors may escape negative selection and may be controlled by a series of peripheral mechanisms that ensure their hyporesponsiveness. Regulatory T cells (Treg) are known to provide a further tier of control in this context. In the thymus and peripheral tissues, DC expand antigen-specific Treg from polyclonal repertoires for peripheral tolerance (Tarbell et al., 2004; Watanabe et al., 2005). In transplantation, there are various pathways of antigen presentation by DC. DC in the grafted tissues mature and migrate into the recipients, where they stimulate alloreactive T cells that bring about graft rejection through the direct pathway of antigen recognition. Recipient DC can also process and present graft antigens and so elicit organ rejection through the indirect pathway. Host DC can replenish grafted tissues (Merad et al., 2002), therefore providing a long-term source of ‘indirect’ antigens to the host immune system. The third pathway of antigen recognition is semi-direct during which recipient DC can acquire donor MHC through cell-to-cell contact for stimulating a T cell response (Jiang et al., 2004). If one could arrest DC in a state where they remained immature and constitutively tolerogenic, or subvert DC to potentiate their tolerance-inducing capacity, then one could aim to switch off potentially alloreactive clones.
The idea of minimizing dosage of toxic drugs and exploiting tolerance mechanisms has been the holy grail of therapeutic immunology since the seminal papers of Medawar and his colleagues in 1953 (Billingham and Boswell, 1953; Billingham et al., 1953; Billingham and Medawar, 1953). Of many experimental strategies that have been investigated for this purpose, the possibility of using ‘expandable’ DC derived from ES cells (esDC) to present antigens for tolerance induction has gained attention from recent research studies (Fairchild et al., 2000; Fairchild et al., 2003). Recently, Senju and colleagues have demonstrated that human esDC can be genetically modified without the use of viral vectors (Senju et al., 2007). Expression vectors for programmed death-ligand 1 (PD-L1) were introduced into human ES cells by electroporation. The transfectants were then directed to differentiate into esDC and by co-culturing with allogeneic T cells, PD-L1-expressing esDC inhibited T-cell proliferation in vitro. This effect was abrogated by the addition of anti-PD-L1 blocking antibodies. Genetic modification of the surface receptors of esDC could, therefore, open new opportunities for treating inflammatory or autoimmune diseases. Blocking co-stimulation and subsequent signaling pathways of DC, such as CD40 and the NF-κB family, by genetic modification might also render DC tolerogenic. Although DC are unable to offer co-stimulation for T cells thereafter, the antigen signal may still be sufficient to tolerise.
Resting DC are known to take up and present self-antigens for tolerance induction (Scheinecker et al., 2002). In this light, many attempts have been made to limit the extent to which DC mature, in the hope of switching their default function from immunity to tolerance. One such approach is mediated through pharmacological modulation. A range of pharmacological agents, such as IL-10, TGF-β (Farquhar et al., 2010; Lan et al., 2006), 1α,25-Dihydroxyvitamin D3 (VD3) (Farquhar et al., 2010; Yates et al., 2007), dexamethasone (Bros et al., 2007) and rapamycin (Turnquist et al., 2007) have been reported to generate “decommissioned” DC. These agents exert various effects on DC, including antigen uptake, maturation, and changes in their expression of cytokines, chemokines and chemokine receptors. There is a hope that generation of such “decommissioned” DC may prevent allograft rejection in an antigen-specific manner. A recent report from our laboratory has, however, demonstrated that there are no shared tolerance-associated transcripts to universally characterize these “decommissioned” DC (Farquhar et al., 2010).
The potential of tolerance induction by pre-treatment of bone marrow-derived DC (bmDC) with IL-10, TGF-β or VD3 has been studied in the TCR transgenic mouse strain A1.RAG-1−/- model (Farquhar et al., 2010; Yates et al., 2007). All T cells of the female A1.RAG-1−/- mice can recognize the male-specific mH antigen, Dby(479–493), presented by H-2Ek (Zelenika et al., 1998). Male bmDC, modulated with IL-10, TGF-β or VD3, could tolerize female A1.RAG-1-/- recipients of male CBA/Ca.RAG-1-/- skin grafts. However, tolerance was not observed in recipients injected with female bmDC, indicating that the response was antigen-specific. CD4+FoxP3+ regulatory T cells (Treg) were found in the tolerated grafts: since there are no naturally-occurring Treg in A1.RAG-1−/- mice, induction of Treg must have occurred locally after injection of the “decommissioned” DC.
However, in many other strain combinations, attempts to induce tolerance by DC alone failed unless combined with other forms of immunosuppression (Morelli and Thomson, 2007). After treatment with IL-10/VD3, rhesus monkey-derived DC were found to induce antigen-specific hyporesponsiveness of T cells when infused into allogeneic recipients in conjunction with co-stimulation blockade (Zahorchak et al., 2007). Pre-treatment of DC with pharmacological agents may, therefore, synergize with other drugs for tolerance induction, minimizing the dosage and toxic side effects associated with current therapies.
4. Immunological privilege of ES cell-derived tissues
Until recently, tissues have been considered only as the passive victims of immune attack. In reality, however, they may play an active a role in determining their own destiny. Certain tissues such as the anterior chamber of the eye, central nervous system, testes and placenta display what has been termed “natural immune privilege” (Medawar, 1948; Mellor and Munn, 2008; Tafuri et al., 1995). Tissues of the placenta are highly sensitive to the detrimental effects of inflammation, whereas others, such as tissues with large mucosal surfaces, are constantly exposed to foreign antigens or organisms under physiological conditions. Some form of immune regulation is, therefore, necessary to override potentially harmful immune activation. The classical paradigm of anterior chamber associated immune deviation (ACAID) (Streilein et al., 2002) taught us that tissues can hold back damaging immune effector functions, albeit to varying degrees. Moreover, spontaneous acceptance of allografts, including liver (Morita et al., 2010) and kidney (Cook et al., 2008), without any added immunosuppression, attests to the existence of tissue-based protective mechanisms. An understanding of how tissues protect themselves from immune damage can provide clues to enable therapeutic amplification of those mechanisms (Driessens et al., 2009; Frey, 2006). Comparable privilege that manifests in certain cancers can also be attributed to the very same active regulatory mechanisms which have sabotaged anti-tumor immunity.
Natural immune privilege is also manifest locally in ES cell-derived tissues (Robertson et al., 2007; Lui et al., 2010). Mouse EB derived from male CBA/Ca mice were spontaneously accepted by female A1.RAG1−/− recipients. Across the same male ‘minor’ difference, however, an anti-CD4 antibody was needed to ensure acceptance of male CBA.RAG1−/− skin grafts in female A1.RAG1−/− recipients. Therefore, unlike conventional tissues such as the skin, ES cell-derived tissues appear to carry a level of natural privilege. Importantly, T cells isolated from spontaneously accepted EB grafts expressed FoxP3, indicating that naïve T cells had been induced towards the regulatory phenotype, since the recipient A1.RAG1−/− mice are normally devoid of Treg (Waldmann and Cobbold, 2001). More recently, we have also demonstrated that ES cell-derived tissues can be naturally accepted across a class-I MHC barrier (Lui et al., 2010). Male CB/K [H-2k, Kb] EB were spontaneously accepted by 50% of male CBA/Ca [H-2k] recipients. Treg appear to be essential for this natural privileged state as their depletion using an anti-CD25 mAb empowers all CBA/Ca recipients to reject CB/K EB. Therefore, by amplifying this ‘innate’ property of ES cells, it might become easier to induce tolerance to tissues across an MHC mismatch. Indeed, EB derived from C57Bl/6 [H-2b] ES cells survived indefinitely in fully allogeneic CBA/Ca [H-2k] recipients treated with mAb directed to CD4 and CD8 (Robertson et al., 2007). By contrast, co-receptor blockade using anti-CD4 and -CD8 mAb was not so efficacious to induce tolerance to MHC-mismatched skin grafts. Therefore, ES cell-derived tissues appear to be more amenable to tolerance induction than conventional allografts. It is possible that long-term acceptance by short-term therapeutic immunosuppressants is simply exploiting and amplifing the natural privilege of ES cell-derived tissues. The finding of CD4+FoxP3+ Treg within tolerated skin grafts (Cobbold et al., 2004; Graca et al., 2002) or naturally accepted EB grafts (Robertson et al., 2007; Lui et al., 2010) raised the possibility that, at least in part, regulation was operating within the protected allograft itself.
5. Proposed mechanisms of natural privilege operating in ES cell-derived tissues
The existence of Treg was first surmised from experiments in which mice that had undergone thymectomy soon after birth, were found to develop spontaneous autoimmune disease (Kong et al., 1989; Sakaguchi et al., 1982). A growing body of evidence shows that there are several types of Treg, the naturally-occurring CD4+FoxP3+ Treg being the best characterized, for their involvement in tolerance (Fontenot et al., 2003). The study of patients with IPEX (immune dysregulation, polyendocrinopathy, enteropathy and X-linked inheritance) syndrome, and the homologous mutant scurfy mouse has uncovered the regulatory function of forkhead transcription factor 3 (FoxP3)-expressing CD4+ Treg. Selective ablation of CD4+FoxP3+ Treg led to multi-system autoimmunity (Kim et al., 2007). The majority of Treg in a normal individual, so-called “natural” Treg, develop in the thymus. Conversion of FoxP3+ Treg from naive CD4+ T cells outside the thymus, so-called “induced” Treg, requires that the T cells receive concomitant signals from both antigen and TGF-β signaling (Chen et al., 2003; Cobbold et al., 2004).
So how do Treg regulate? Apart from polarizing naïve CD4+ T cells to “induced” Treg, Treg can also decommission antigen-presenting cells (APC). Such APC then become less activated or actively anti-inflammatory in the privileged microenvironment. Fresh cohorts of naïve T cells, constantly exposed to antigens in the absence of danger signals and costimulation from such DC are then more likely to become tolerised. In particular, Treg modulate macrophages by direct cell contact and release of IL-10 (Tiemessen et al., 2007). After co-culture with Treg in vitro, human monocytes or macrophages display typical features of alternatively activated macrophages (AAM), accompanied with up-regulated expression of CD206 (mannose receptor), enhanced phagocytic capacity, increased production of CCL18 and reduced expression of HLA-DR. These macrophages are anti-inflammatory and are capable of mediating tumor promotion (Sica et al., 2006).
Treg can also suppress effector activity of CD8+ T cells in models of transplantation tolerance and cancer. Our laboratory has demonstrated that coreceptor-induced tolerance did not preclude antigen-reactive CD8+ T cells from proliferating. However, such CD8+ T cells had impaired effector functions and were unable to produce IFN-γ, to become cytotoxic and to reject grafts (Lin et al., 2002; Lui et al., 2010). In a model of murine colon carcinoma, CD8+ T cells expanded to the same extent and produced similar levels of IFN-γ in the presence or absence of tumor-specific Treg (Chen et al., 2005). However, these Treg abrogated CD8+ T cell-mediated tumor rejection by specifically suppressing the cytotoxicity of expanded CD8+ T cells. These data indicate that even late stages of T-cell differentiation are amenable to control by Treg. Nevertheless, it is not yet clear how Treg exert their suppressive functions in tissues, but physical contact with immune cells and/or production of immunosuppressive cytokines including IL-10 and TGF-β are likely just the tip of the iceberg of the range of mechanisms that have been implicated (Tang and Bluestone, 2008). Figure 2 below summarizes mechanisms of immune regulation by Treg.
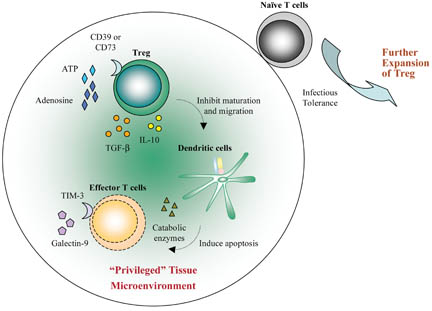
Treg migrate to the grafted tissue. Activated Treg convert ATP released by inflamed tissues to adenosine via the ectoenzymes CD39 and CD73. Local adenosine could contribute to the initial “privileged” microenvironment. Treg also secrete TGF-β and IL-10 which inhibit the maturation and migration of dendritic cells (DC). These “decommissioned” DC can secrete catabolic enzymes for depletion of essential amino acids (EAA) and, therefore, induce apoptosis of effector T cells. Moreover, the “privileged” tissues could release pro-apoptotic galectin-9 which binds to TIM-3 expressed by effector T cells such as Th1 and Th17. This further amplifies the immunosuppressive microenvironment. Each of these components within the graft can further reinforce the local anti-inflammatory state such that any naive T cell, migrating into this area, will be converted to induced Treg (iTreg) through infectious tolerance. The iTreg then expand and further suppress immunity in the “privileged” microenvironment.
5.1. Infectious tolerance through TGFβ signaling
Dominant tolerance depends on extrinsic regulation through Treg. When attempts were made to break the tolerant state in skin grafts, established with administration of anti-CD4 and -CD8 mAb, by adoptive transfer of naïve T cells into the tolerised recipients, this second cohort of T cells did not break tolerance but became tolerant in its own right (Qin et al., 1989; Waldmann et al., 2008). In fact, tolerance mechanisms by Treg require continued exposure to Ag and continued recruitment of new Treg, a process known as ‘infectious tolerance’ (Cobbold et al., 2004). Infectious tolerance, therefore, allows the immune system to perpetuate life-long regulation through polarization of naïve T cells to antigen-specific Treg. Such conversion requires TGF-β (Cobbold et al., 2004; Daley et al., 2007) and IL-2 for its induction (Davidson et al., 2007).
Unlike the thymic-derived “natural” Treg, which can develop and be fully functional in the absence of TGF-β signaling (Fahlen et al., 2005), TGF-β is essential for induction of FoxP3 expression and de novo differentiation of “induced” CD4+ Treg (Cobbold et al., 2004; Kretschmer et al., 2005; Robertson et al., 2007; Tone et al., 2008). Long-term tolerance of male CBA.RAG-1−/- skin grafts in female A1.RAG-1−/- recipients after short pulses of the non-depleting anti-CD4 mAb is dependent on TGF-β signaling (Cobbold et al., 2004; Daley et al., 2007). Such an effect was prevented using TGF-β-neutralizing mAb in vivo. For EB grafts, Robertson and colleagues have also shown that EB could be spontaneously accepted across a minor histocompatibility barrier when male CBA/Ca EB were transplanted into female A1.RAG-1−/- mice. Although this outcome could, in principle, be explained by some form of immunological ignorance, it was observed that CD4+FoxP3+ Treg had been generated within the accepted tissues without any exogenous immunosuppression. Accepted tissues showed expression of TGF-β2 and some of the infiltrating CD4+ T cells demonstrated phosphorylation of SMAD2/3, evidence of signaling through TGF-β (Derynck and Zhang, 2003; Shi and Massague, 2003; Tone et al., 2008). Moreover, no conversion of “induced” Treg could be observed when recipients expressed a dominant negative TGFβRII receptor (dnTGFβRII) on their T cells, where the truncated intracellular kinase domain of the TGFβRII failed to trigger downstream signaling events. TGF-β signaling is, therefore, required to maintain long-term acceptance of allografts.
5.2. Infectious tolerance through mTOR signaling
It has been reported that rat and human mesenchymal stem cells (MSC) contribute to local immunosuppression, in part, through induction of inducible nitric oxide synthase (iNOS) (Chabannes et al., 2007). Injection of rat MSC in vivo significantly delayed heart allograft rejection. However, treatment with iNOS inhibitor, aminoguanidine, totally reversed the protective effect of MSC. Another enzyme which contributes to the immunosuppressive effect of MSC is indoleamine 2,3-dioxygenase (IDO) (Jones et al., 2007). Treatment with IDO inhibitor, 1-methyl-dl-tryptophan (1-MT), attenuated the effect of placenta-derived MSC in inhibiting allogeneic T-cell proliferation.
Both iNOS and IDO are catabolic enzymes which consume the essential amino acids (EAA) arginine and tryptophan, respectively. In fact, IDO has been regarded as an immunosuppressive enzyme. This role for IDO was first demonstrated by Munn et al. who showed that it was required for maintaining tolerance to paternal antigens expressed by the fetus (Munn et al., 1998). Specific inhibition of IDO activity by 1-MT in the placenta induced rejection of semi-allogeneic, but not syngeneic, concepti in normal mice. IDO induction in APC via CTLA4 ligation of CD80/CD86 is also thought to represent an important effector pathway for induction of Treg (Mellor et al., 2004; Mellor and Munn, 2004; Puccetti and Grohmann, 2007), which was shown to be important for spontaneous acceptance of renal allografts (Cook et al., 2008). For ES cell-derived tissues, we have also observed expression of the Ido gene in naturally accepted EB grafts 2–4 weeks after transplantation. There was, however, no reduction in rate of graft survival after transplanting the tissues in IDO−/− recipients (unpublished data). Indeed, IDO deficient mice did not show any increase in rate of spontaneous abortion of allogeneic concepti (Baban et al., 2004). IDO−/− mice also do not show any gross immunological phenotype. Therefore, local consumption of multiple EAA would seem to represent a redundant and, therefore, functionally robust system for maintaining immune privilege.
More recently, evidence is accumulating to demonstrate that the conversion of naïve T cells to “induced” Treg also depends on inhibition of the mammalian target of rapamycin (mTOR) pathway (Cobbold et al., 2009; Haxhinasto et al., 2008; Sauer et al., 2008). Our group has shown that antigen-specific Treg induce, within skin grafts and DC, expression of catabolic enzymes which consume at least five different EAA, including arginine, histamine, histidine, serotonin and tryptophan (Cobbold et al., 2009). Effector T cells fail to proliferate in response to antigens when any one, or more, of these EAA are limiting, which is associated with reduced mTOR signaling. Inhibition of the mTOR pathway by limiting EAA, or by specific inhibitors, also induces Treg-specific FoxP3, which depends on both T cell receptor activation and synergy with TGF-β.
5.3. Expression of immunoregulatory molecules
In addition to TGF-β and IL-10 (Daley et al., 2007; Hara et al., 2001), galectin-9 has been recently reported to be a target for immune regulation. It is a ligand of Tim-3 (T cell Ig and mucin-3) which is capable of inducing apoptosis of Tim-3-expressing Th17 (Seki et al., 2008) and Th1 (Klibi et al., 2009) cells. Galectin-9 ameliorated murine collagen-induced arthritis (CIA) by suppressing the generation of Th17 cells and promoting induction of Treg (Seki et al., 2008). In the privileged microenvironment of tumors such as human nasopharyngeal cancer, secreted galectin-9 has also been implicated in Th1 immunosuppression (Klibi et al., 2009). In transplantation, blocking the galectin-9/Tim-3 pathway prolonged survival of allogeneic skin grafts induced by CD4+ Tregs in vivo (Wang et al., 2009).
Activated Treg can convert ATP released by inflamed tissues to adenosine via the ectoenzymes CD39 and CD73 (Deaglio et al., 2007). Local adenosine concentration, therefore, contributes to the initial immunosuppressive milieu. We have also observed protein expression of galectin-9 and CD73 in naturally accepted EB grafts 4 weeks after transplantation (unpublished data). Nevertheless, the roles of tissue expression of these “immunoregulatory” molecules are yet to be elucidated. It might be possible that ES cell-derived tissues exploit several pathways for maintaining immune privilege.
5.4. Linked suppression
The finding of linked suppression suggests that lymphoid tissues may not be the major site for immune regulation. Mice of strain A, tolerised to grafts derived from strain B, would reject grafts derived from strain C, but not from (BxC)F1 donors (where the terms A, B and C denote histoincompatible mouse strains) (Chai et al., 2004; Davies et al., 1996; Waldmann et al., 2008). Both strains B and C differ across the whole MHC locus. Therefore, linked suppression demonstrates a mechanism of immune regulation in which tolerance could be induced to third-party antigens in the same tissue. A tissue microenvironment influenced by Treg may take on a reinforcing role to stabilize T cells with a tendency for regulation. Both the gut and tumors, for instance, constitute a very stabilizing microenvironment for immune regulation.
6. Induction of donor-specific tolerance for ES cell-derived tissues
Recently, there are antibody-based clinical trials in autoimmune diseases where short-term therapy promotes long-term tolerance with minimum drug intervention. Although these trials have been largely confined to a few diseases such as Type I diabetes and multiple sclerosis (Coles et al., 2008; Coles et al., 2006; Herold et al., 2005; Keymeulen et al., 2005) (both types of trial are currently in Phase III), they may still serve to offer a tolerising protocol for promoting acceptance of ES cell-derived tissues. In fact, antibodies against T cell co-receptors (Cobbold et al., 2004; Daley et al., 2007) and costimulatory molecules (Linsley and Nadler, 2009) are all capable of recruiting Treg, favoring regulatory mechanisms in vivo. As mentioned above, a short course of treatment with anti-CD4 and -CD8 antibodies alone also permitted the indefinite survival of fully allogeneic ES cell-derived tissues in vivo (Robertson et al., 2007; Lui et al., 2010).
In transplantation of skin grafts, the regime of coreceptor blockade also exploits short-term treatment for long-term tolerance of allografts in vivo (Waldmann et al., 2008). A combination of anti-CD4 and -CD8 antibodies could achieve transplantation tolerance across mismatches for multiple minor histocompatibility antigens (Qin et al., 1990) and for full MHC disparity when combined with costimulation blockade in the form of antibodies to CD154 (Graca et al., 2004; Honey et al., 1999). In one study, a short course of anti-CD4 antibody therapy could induce tolerance to aggregated human gammaglobulin (HGG), normally very immunogenic in the mouse (Qin et al., 1990). Mice tolerized to HGG remained indefinitely but specifically unresponsive to HGG on many rechallenges over a long period of time. However, tolerance was lost if the first rechallenge was delayed long enough after the antigen had been cleared. Therefore, a sustained supply of antigen is needed to maintain tolerance in the absence of further immunosuppression. In transplantation, the organ itself or tissues to be transplanted would be a constant source of such antigens for maintaining the tolerant status (Waldmann et al., 2008). Notably, if the rechallenge were delayed, the “tolerant” T cell population would regain its ability to reject. This suggests that the potential for generating effector T cells still exists in the tolerant hosts, but is controlled by antigen-dependent regulation, for example, through Treg. In a similar vein, if more in vivo data can demonstrate their efficacy, any therapeutic vaccination that can expand antigen-specific Treg, or drugs that can enhance FoxP3+-directed regulation, may also prove effective at promoting graft survival of allogeneic ES cell-derived tissues. Such strategies reinforce the tissue's natural capacity for immune privilege by amplifying regulation through Treg.
There are also a number of cell surface receptors and ligands, expressed on lymphocytes, APC or the localized tissue, that are capable of delivering or accepting extrinsic inhibitory signals to prevent autoimmune diseases. These include T-cell immunoglobulin mucin family (TIM) members (Sanchez-Fueyo et al., 2003), cytotoxic T-lymphocyte protein (CTLA4) (Walunas et al., 1994) and programmed cell death receptor (PD1) and its ligand PDL1 (Francisco et al., 2009; Keir et al., 2008). Furthermore, inhibitory (ITIM) receptors (Daeron et al., 1995; Van den Herik-Oudijk et al., 1995) such as FcγRII, and members of sialic acid binding Ig-like lectins (Siglecs) (Crocker, 2002) perform similar functions on B-cells. The regulatory power of CTLA4, for example, is now being exploited clinically in the form of an immunoglobulin Fc-based fusion protein (Linsley and Nadler, 2009).
7. Conclusion
The characterization of syndromes in man such as APECED and IPEX have been instrumental in highlighting the presence of mechanistic hierarchies for tolerance in the primary lymphoid organs as well as the periphery. Lessons from ACAID and tumors have also taught us about mechanisms for privilege in a localized microenvironment. In fact, induction of Treg, “decommissioned” APC, catabolic enzymes for EAA, co-inhibitory molecules and anti-inflammatory cytokines (such as TGF-β and IL10) are all exhibited in protection of cancers against the immune system. Therefore, strategies can be made to target these various properties of tissue privilege for enhancing the efficacy of tolerance induction to minimize side effects from conventional immunosuppression.
Acknowledgements
K.O.L. holds a Croucher Foundation Fellowship and is currently affiliated with the Cardiovascular Research Center of Harvard Stem Cell Institute. The authors declare no conflicts of interests.

